推荐产品
产品名称
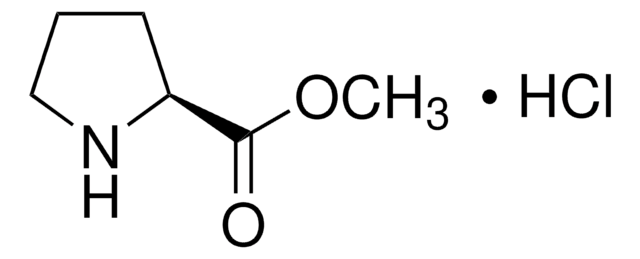
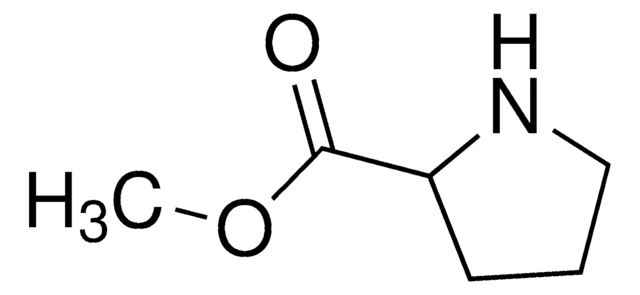
L-脯氨酰胺, 98%
品質等級
化驗
98%
光學活性
[α]20/D −106°, c = 1 in ethanol
反應適用性
reaction type: solution phase peptide synthesis
mp
95-97 °C (lit.)
應用
peptide synthesis
SMILES 字串
NC(=O)[C@@H]1CCCN1
InChI
1S/C5H10N2O/c6-5(8)4-2-1-3-7-4/h4,7H,1-3H2,(H2,6,8)/t4-/m0/s1
InChI 密鑰
VLJNHYLEOZPXFW-BYPYZUCNSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Hidenobu Komeda et al.
European journal of biochemistry, 271(8), 1465-1475 (2004-04-07)
An amidase acting on (R,S)-piperazine-2-tert-butylcarboxamide was purified from Pseudomonas azotoformans IAM 1603 and characterized. The enzyme acted S-stereoselectively on (R,S)-piperazine-2-tert-butylcarboxamide to yield (S)-piperazine-2-carboxylic acid. N-terminal and internal amino acid sequences of the enzyme were determined. The gene encoding the S-stereoselective
Kazuhiko Mitsui et al.
Chemical communications (Cambridge, England), (22)(22), 3261-3263 (2009-07-10)
Dendritic effects on both the enantioselectivity and diastereoselectivity of the direct aldol reaction were observed for pyridine-2,6-dicarboxamide dendrons terminated with L-prolinamides.
Yasuhiro Goto et al.
Journal of medicinal chemistry, 49(3), 847-849 (2006-02-03)
A focused library approach identifying novel leads to develop a potent ORL1 antagonist is described. Beginning from a compound identified by random screening, an exploratory library that exhibited a diverse display of pharmacophores was designed. After evaluating ORL1 antagonistic activity
Sampak Samanta et al.
Organic letters, 7(23), 5321-5323 (2005-11-05)
[reaction: see text] The catalytic activity of the prolinamide-type catalysts may be improved by introducing additional prolinamide moiety into the catalyst, while the enantioselectivity can still be maintained or further improved. A C2-symmetric bisprolinamide with two prolinamide moieties has been
Highly enantioselective strecker reaction of ketoimines catalyzed by an organocatalyst from (S)-BINOL and L-prolinamide.
Zongrui Hou et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(15), 4484-4486 (2008-04-11)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门