所有图片(1)
About This Item
经验公式(希尔记法):
C8H8O2
CAS号:
分子量:
136.15
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.532 (lit.)
bp
199-200 °C (lit.)
密度
1.135 g/mL at 25 °C (lit.)
SMILES 字串
Cc1ccc2OCOc2c1
InChI
1S/C8H8O2/c1-6-2-3-7-8(4-6)10-5-9-7/h2-4H,5H2,1H3
InChI 密鑰
GHPODDMCSOYWNE-UHFFFAOYSA-N
應用
3,4-(Methylenedioxy)toluene can be used as a cation scavenger for the selective cleavage of 4-(3,4-dimethoxyphenyl)benzyl (DMPBn) ethers affording the corresponding deprotected products in the presence of trifluoroacetic acid (TFA) in anhydrous CH2Cl2.
It can also be used as a starting material to prepare:
It can also be used as a starting material to prepare:
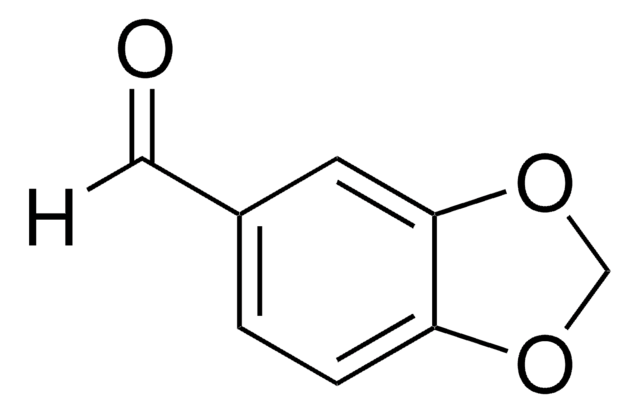
- 1,3-Benzodioxole-5-carboxaldehyde via photooxidation using CBr4.
- 6-Methyl-1,3-benzodioxole-5-carboxaldehyde by treating with dichloromethylmethyl ether and TiCl4.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
168.8 °F - closed cup
閃點(°C)
76 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Visible light photocatalysis with CBr 4: a highly selective aerobic photooxidation of methylarenes to aldehydes
Shubhangi T, et al.
Royal Society of Chemistry Advances, 6(18), 14547-14551 (2016)
Gregory M Rankin et al.
The Journal of organic chemistry, 78(11), 5264-5272 (2013-05-18)
A reliable reagent system for the cleavage of 4-(3,4-dimethoxyphenyl)benzyl (DMPBn) ethers under acidic conditions has been established. Treatment of DMPBn-protected mono- and pseudodisaccharides with TFA in anhydrous CH2Cl2 and 3,4-(methylenedioxy)toluene as a cation scavenger resulted in the selective cleavage of
Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues
Aslam SN, et al.
Tetrahedron, 62(17), 4214-4226 (2006)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



![苯并[h]喹啉 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)

