推荐产品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.447 (lit.)
bp
158-161 °C (lit.)
密度
1.656 g/mL at 25 °C (lit.)
官能基
fluoro
nitro
SMILES 字串
[O-][N+](=O)c1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F5NO2/c7-1-2(8)4(10)6(12(13)14)5(11)3(1)9
InChI 密鑰
INUOFQAJCYUOJR-UHFFFAOYSA-N
一般說明
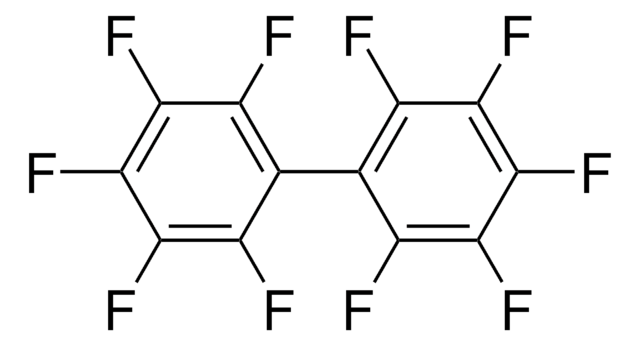
Electron attachment to pentafluoronitrobenzene has been studied in the energy range 0-16eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. The electroreduction of pentafluoronitrobenzene in dimethylformamide solution results in the formation of the dimer, octafluoro-4,4′-dinitro-biphenyl.
應用
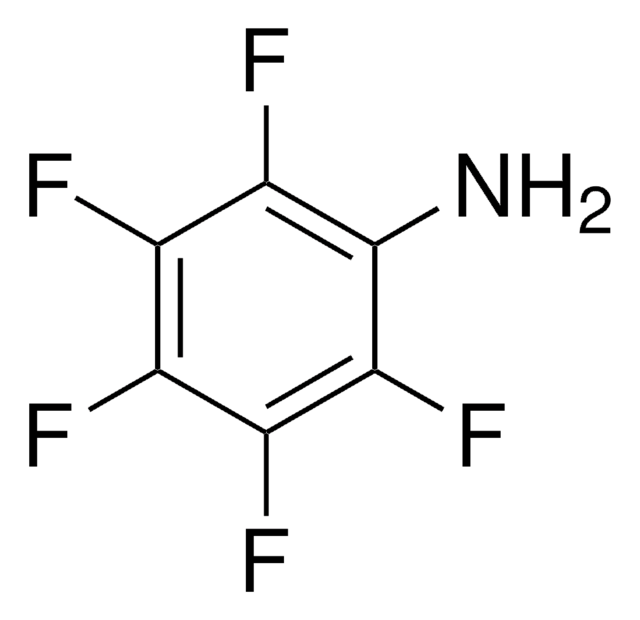
Pentafluoronitrobenzene has been used in the preparation of p-azidotetrafluoroaniline, a new photoaffinity reagent.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
195.8 °F - closed cup
閃點(°C)
91 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Judith Langer et al.
Physical chemistry chemical physics : PCCP, 10(11), 1523-1531 (2008-03-11)
Electron attachment to pentafluorobenzonitrile (C(6)F(5)CN) and pentafluoronitrobenzene (C(6)F(5)NO(2)) is studied in the energy range 0-16 eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. We find that pentafluoronitrobenzene exclusively generates fragment anions via
Voltammetry under high mass transport conditions. The application of the high speed channel electrode to the reduction of pentafluoronitrobenzene.
Coles BA, et al.
Journal of Electroanalytical Chemistry, 411(1), 121-127 (1996)
K A Chehade et al.
The Journal of organic chemistry, 65(16), 4949-4953 (2000-08-24)
p-Azidotetrafluoroaniline (1) was synthesized in 65-73% yield by two different methods employing a stable carbamate intermediate. The first method trapped the intermediate isocyanate generated via a modified Curtius rearrangement with 2-methyl-2-propanol or 2-(trimethylsilyl)ethanol to form the stable carbamates 2d and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门