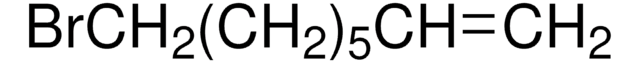推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.463 (lit.)
bp
126-127 °C/765 mmHg (lit.)
密度
1.258 g/mL at 25 °C (lit.)
官能基
alkyl halide
allyl
bromo
儲存溫度
2-8°C
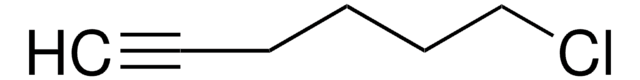
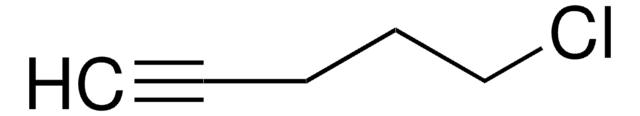
SMILES 字串
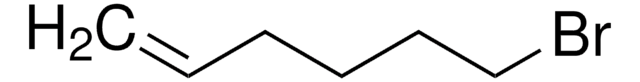
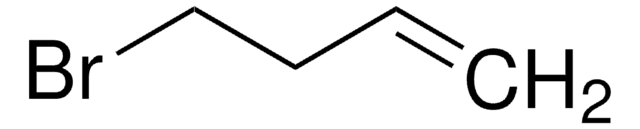
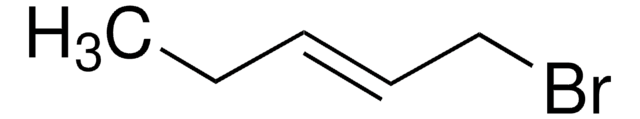
BrCCCC=C
InChI
1S/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
InChI 密鑰
LPNANKDXVBMDKE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
5-溴-1-戊烯用于立体选择性合成 7α-(3-羧丙基) 雌二醇。用于制备具有硫代糖苷键的唾液酸的硫代乙酸酯 11。它还被用作最近合成 DL-组织毒素和含二苯甲酮的脂肪酸的起始材料。
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
87.8 °F - closed cup
閃點(°C)
31 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Jun-Ichi Sakamoto et al.
Bioorganic & medicinal chemistry letters, 17(3), 717-721 (2006-11-11)
An efficient synthesis of a series of carbosilane dendrimers uniformly functionalized with alpha-thioglycoside of sialic acid was accomplished. The results of a preliminary study on biological responses against influenza virus sialidases using thiosialoside clusters showed that some of the glycodendrimers
Yonghong Gan et al.
The Journal of organic chemistry, 71(25), 9487-9490 (2006-12-02)
Syntheses of new benzophenone-containing fatty acids (FABPs) 1, 5, and 6 and a new route to FABP 3 are described. Combined with the known 2 and 4, these FABPs comprise a set of photoactivatable fatty acid analogues with the crosslinking
M Adamczyk et al.
Steroids, 62(12), 771-775 (1998-01-22)
Alkylation of 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-1,3,5(10) estratriene-6-one (2) with 5-bromo-1-pentene using NaHMDS in THF afforded 3,17 beta-bis(2-trimethylsilyl)ethoxymethyl-7-alpha-(4'pentenyl)-1,3,5(10) estratriene-6-one (3) in excellent stereoselectivity (> 95% epimeric excess). Functionalization of the side chain in compound 3 was accomplished via ozonolysis, oxidation and esterification to
Maheswaran S Karatholuvhu et al.
Journal of the American Chemical Society, 128(39), 12656-12657 (2006-09-28)
The synthesis of (+/-)-histrionicotoxin has been achieved in just nine steps using a two-directional synthesis strategy. Key reactions include a two-directional cross-metathesis, a tandem oxime formation/Michael addition/1,4-prototopic shift/[3 + 2]-cycloaddition cascade, a selective Z,Z-bisenyne formation, and a one-pot N-O and
Maher A Qaddoura et al.
International journal of molecular sciences, 10(11), 4772-4788 (2010-01-21)
Several divinylic mesogenic monomers were synthesized based on coupling the monomer 4-(4-pentenyloxy)benzoic acid with chlorohydroquinone, 2,5-dihydroxy- acetophenone, methylhydroquinone or 2-methoxyhydroquinone. This resulted in novel mesogens of phenylene esters with different lateral substituent groups. The effect of the lateral substituent group
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门