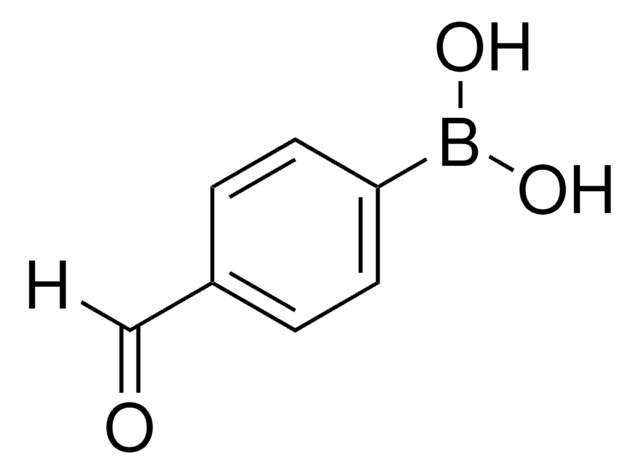
推荐产品
品質等級
化驗
97%
形狀
powder
mp
173-175 °C (lit.)
溶解度
methanol: soluble 100 mg/mL, clear to slightly hazy, colorless to very faintly brown(lit.)
官能基
aldehyde
carboxylic acid
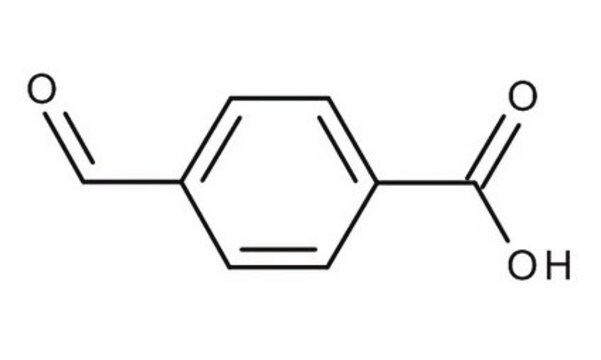
SMILES 字串
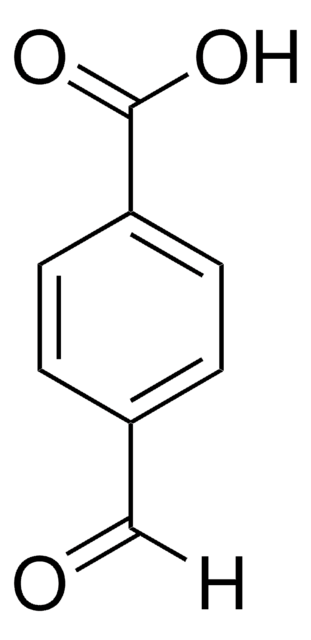
[H]C(=O)c1cccc(c1)C(O)=O
InChI
1S/C8H6O3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-5H,(H,10,11)
InChI 密鑰
UHDNUPHSDMOGCR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
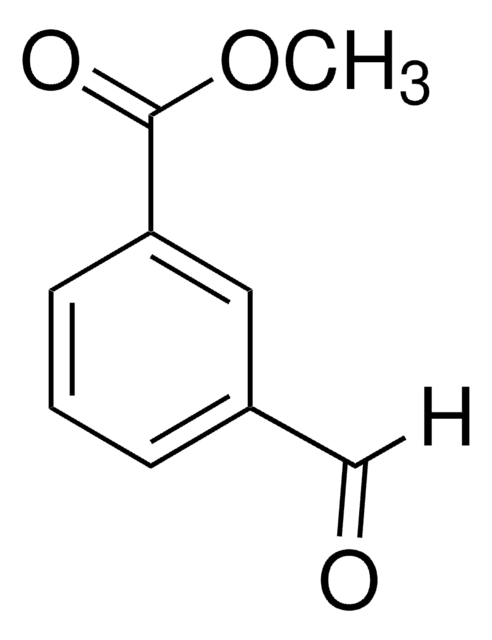
3-甲酰苯甲酸是一种极性芳香醛,常用于还原合成3-羟甲基苯甲酸。
應用
3-甲酰基苯甲酸已被用于合成:
- 通过Ugi 4中心3组分反应的双环顺式-2-氮杂环丁酮衍生物合成
- 带有会聚羟基的甾族超结构封端的卟啉
- 3-[(4-氨基-1,2-二氢-1-氧代-2-苯基-1,2,4-三唑[4,3-a]喹喔啉-6-基)氨基]甲基苯甲酸
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Synthesis of Alicyclic-lactams via the Ugi Reaction on a Solid Support.
Gedey S, et al.
Letters in Organic Chemistry, 1(3), 215-220 (2004)
Suhman Chung et al.
Nature chemical biology, 5(6), 407-413 (2009-04-28)
The linking together of molecular fragments that bind to adjacent sites on an enzyme can lead to high-affinity inhibitors. Ideally, this strategy would use linkers that do not perturb the optimal binding geometries of the fragments and do not have
Vittoria Colotta et al.
Bioorganic & medicinal chemistry, 11(24), 5509-5518 (2003-12-04)
In previous papers (Colotta, V. et al. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 39. Colotta, V. et al. J. Med. Chem. 2000, 43, 1158) we reported the synthesis and binding affinity at bovine (b) A(1) and A(2A) and human
Synthesis, binding properties and self-functionalization of a steroid-capped porphyrin.
Richard P and Jeremy KM.
Journal of the Chemical Society. Chemical Communications, 8, 574-577 (1991)
Steven R Inglis et al.
Journal of medicinal chemistry, 52(19), 6097-6106 (2009-09-08)
Penicillin binding proteins (PBPs) catalyze steps in the biosynthesis of bacterial cell walls and are the targets for the beta-lactam antibiotics. Non-beta-lactam based antibiotics that target PBPs are of interest because bacteria have evolved resistance to the beta-lactam antibiotics. Boronic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门