推荐产品
化驗
98%
形狀
solid
mp
151-154 °C (lit.)
溶解度
alcohol: soluble(lit.)
water: soluble(lit.)
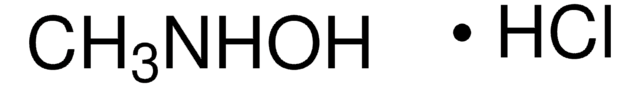
SMILES 字串
Cl.CON
InChI
1S/CH5NO.ClH/c1-3-2;/h2H2,1H3;1H
InChI 密鑰
XNXVOSBNFZWHBV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
甲氧胺盐酸盐与人抗凝血酶III-凝血酶复合物结合,形成无活性的凝血酶。
應用
甲氧基胺盐酸盐被用作制备 O-甲基肟的试剂。它还被用于由醛或酮合成 O-甲基肟。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
The Journal of Organic Chemistry, 59, 1492-1492 (1994)
Synthesis, 849-849 (1992)
M O Longas et al.
The Biochemical journal, 189(3), 481-489 (1980-09-01)
1. Cleavage of the human antithrombin III--thrombin complex with [14C]methoxyamine hydrochloride results in inactive thrombin and 14C-labelled antithrombin III. 2. Discontinuous polyacrylamide-gel electrophoresis of the reduced dissociation fragments of the complex in the presence of sodium dodecyl sulphate reveals two
Jean-Philippe Mevy et al.
Plants (Basel, Switzerland), 9(9) (2020-09-10)
Global change scenarios in the Mediterranean basin predict a precipitation reduction within the coming hundred years. Therefore, increased drought will affect forests both in terms of adaptive ecology and ecosystemic services. However, how vegetation might adapt to drought is poorly
Edouard Godineau et al.
Organic letters, 8(21), 4871-4874 (2006-10-06)
[reaction: see text] A formal [2+2+2] process has been devised that allows the stereocontrolled formation of ring-fused piperidines from allylsilanes possessing an oxime moiety. The cascade involves an intermolecular radical addition of an alpha-iodoacetate onto an allylsilane double bond, which
商品
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门