推荐产品
品質等級
產品線
ReagentPlus®
化驗
99%
形狀
powder
mp
134-135 °C (lit.)
溶解度
ethanol: soluble 50 mg/mL, clear, yellow to orange
SMILES 字串
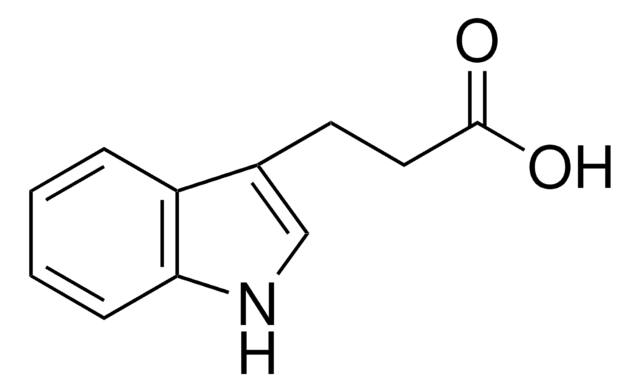
OC(=O)CCc1c[nH]c2ccccc12
InChI
1S/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14)
InChI 密鑰
GOLXRNDWAUTYKT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-吲哚丙酸是错误折叠的β-淀粉样蛋白(Abeta)聚集的一种有效抑制剂。通过三组分一锅法进行3-吲哚丙酸的组装已被报道。
應用
反应物用于制备:
- 独脚金内酯的荧光类似物
- 抗肿瘤剂
- 黑皮质素受体配体
- 免疫抑制剂
- 丙肝病毒抑制剂
- 组胺H4受体激动剂
- NR2B/NMDA受体拮抗剂
- CB1拮抗剂用于治疗肥胖症免疫抑制剂
- 抗细菌剂
- TGF-β受体结合抑制剂
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Mauro F A Adamo et al.
Organic letters, 9(2), 303-305 (2007-01-16)
A three-component one-pot procedure (3-MC) was developed to assemble 3-indolepropionic acids from commercially available materials. This new methodology affords the title compounds in high yields and without the use of chromatography. [reaction: see text].
Xun Cheng et al.
Analytical chemistry, 77(21), 7012-7015 (2005-11-01)
Alzheimer's disease is the most common cause of the loss of cognitive function among the elderly, and the aggregation and deposition of misfolded beta-amyloid protein (Abeta) contribute to this progressive central nervous system decline. Therefore, compounds that inhibit or even
Andrew W Woodward et al.
Plant physiology, 144(2), 976-987 (2007-04-24)
The ubiquitin-like protein RELATED TO UBIQUITIN (RUB) is conjugated to CULLIN (CUL) proteins to modulate the activity of Skp1-Cullin-F-box (SCF) ubiquitylation complexes. RUB conjugation to specific target proteins is necessary for the development of many organisms, including Arabidopsis (Arabidopsis thaliana).
B Poeggeler et al.
Brain research, 815(2), 382-388 (1999-01-08)
The hydroxyl radical scavenging activity of indole-3-propionate was evaluated by kinetic competition studies with the hydroxyl radical trapping reagent 2,2'-azino-bis-(3-ethyl-benz-thiazoline-6-sulfonic acid) (ABTS) and by measuring hydroxyl radical-initiated lipid peroxidation in the rat striatum. Using ABTS, the indole was shown to
K L Borden et al.
European journal of biochemistry, 202(2), 459-470 (1991-12-05)
The antirepressor indole 3-propanoate has been shown by X-ray crystallography to bind in a different orientation compared with the natural corepressor for the tryp repressor, L-tryptophan (Lawson, C.L. & Sigler, P. B. (1988) Nature 333, 869-871). This suggests a simple
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门