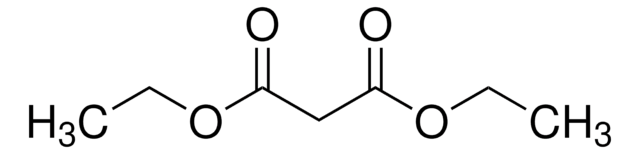
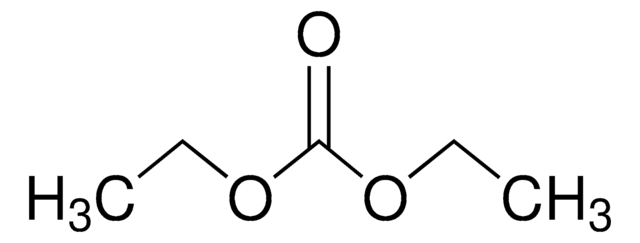
推荐产品
化驗
98%
形狀
solid
mp
92-96 °C (lit.)
溶解度
dioxane: soluble 5%, clear to very slightly hazy, colorless to faintly yellow
官能基
ester
ketal
儲存溫度
2-8°C
SMILES 字串
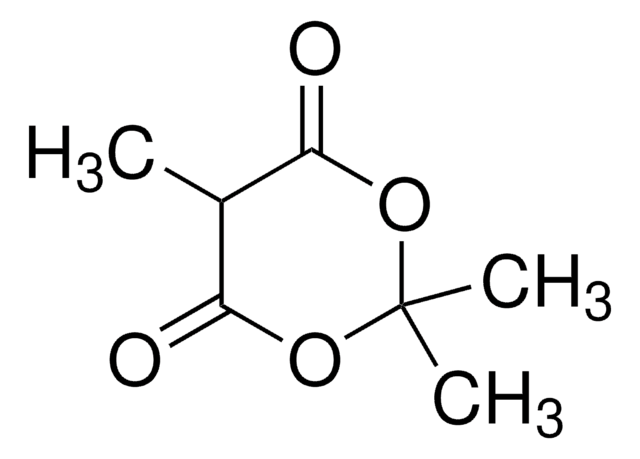
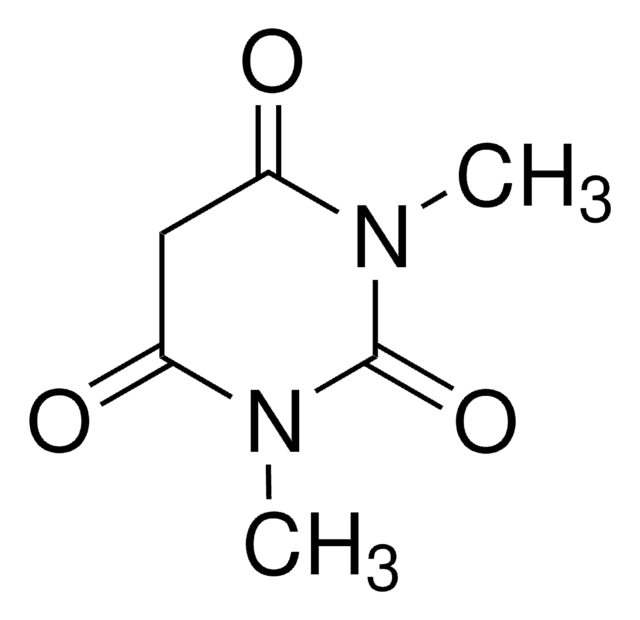
CC1(C)OC(=O)CC(=O)O1
InChI
1S/C6H8O4/c1-6(2)9-4(7)3-5(8)10-6/h3H2,1-2H3
InChI 密鑰
GXHFUVWIGNLZSC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
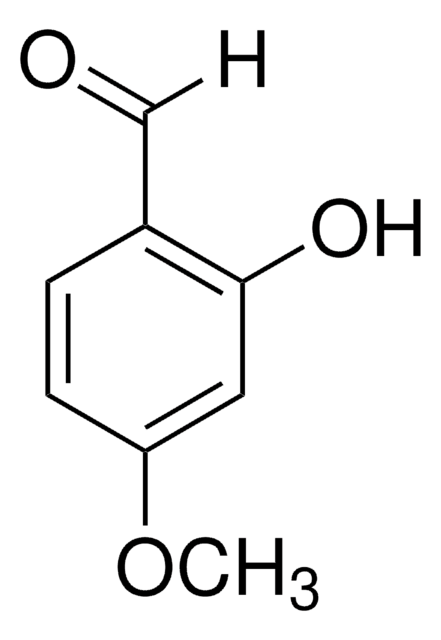
2,2-二甲基-1,3-二恶烷-4,6-二酮(米氏′酸)广泛用于有机合成,特别是对于多种CC键形成,因为它具有足够的酸性 (pKa 4.83) 和空间刚性。醛与米氏′酸之间的Knoevenagel缩合反应在离子液体中加速。
米氏′酸被用作合成杂环的有价值的起始原料和有机合成反应的中间体。
米氏′酸被用作合成杂环的有价值的起始原料和有机合成反应的中间体。
應用
2,2-二甲基-1,3-二恶烷-4,6-二酮用于合成:
- 大环 β-酮内酯
- 4-吡啶基取代的杂环
- 2-取代吲哚
- 异秦皮啶。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Aaron M Dumas et al.
Accounts of chemical research, 43(3), 440-454 (2009-12-17)
Meldrum's acid (2,2-dimethyl-1,3-dioxane-4,6-dione) is a molecule with a unique history, owing to its originally misassigned structure, as well as a unique place among acylating agents, owing to its high acidity and remarkable electrophilicity. In this Account, we outline the work
Songlei Zhu et al.
Molecules (Basel, Switzerland), 17(12), 13856-13863 (2012-11-24)
A series of 4-aryl-6-methyl-3,4-dihydro-2H-pyrano[3,2-c]quinolin-2,5(6H)-diones were synthesized via the three-component reactions of aromatic aldehydes, 4-hydroxy-1-methylquinolin-2(1H)-one, and Meldrum's acid catalyzed by L-proline. The structures of the products were identified by spectroscopic analysis. A mechanism for this three-component reaction catalyzed by L-proline was
Davood Nematollahi et al.
Chemical & pharmaceutical bulletin, 58(1), 23-26 (2010-01-05)
Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid as a nucleophile in aqueous solution has been studied in detail by means of cyclic voltammetry and controlled potential coulometry. The results indicate that the o-benzoquinone derived from catechols participates
The synthesis of β-keto lactones via cyclization of β-keto ester dianions or the cyclization of Meldrum's acid derivatives.
Lermer L, et al.
Canadian Journal of Chemistry, 70(5), 1427-1445 (1992)
New multicomponent domino reactions (MDRs) in water: highly chemo-, regio-and stereoselective synthesis of spiro {[1, 3] dioxanopyridine}-4, 6-diones and pyrazolo [3, 4-b] pyridines.
Ma N, et al.
Green Chemistry, 12?(8), 1357-1361 (2010)
商品
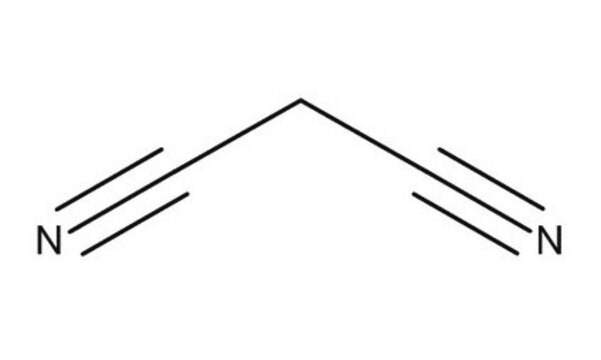
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门