推荐产品
形狀
liquid
品質等級
反應適用性
reagent type: reductant
濃度
1.0 M in THF
密度
0.865 g/mL at 25 °C
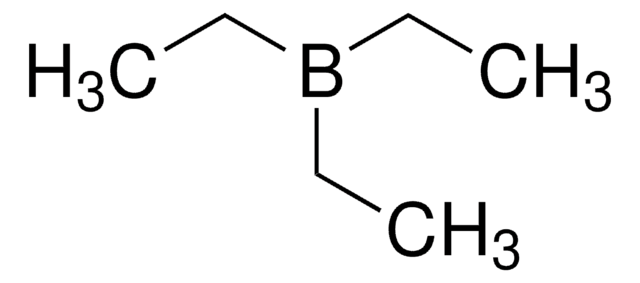
SMILES 字串
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI 密鑰
LALRXNPLTWZJIJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- 醛的烯丙基化
- 醛的脱羧 C-C 键断裂反应
- 铼氢化物/硼路易斯酸共催化烯烃加氢反应
- 不饱和肟醚的区域选择性羟基烷基化
它可作为反应物用于 N -杂环卡宾硼烷 的烷基溴自由基还原反应和四甲基铵三烷基苯硼酸盐的合成。
- 醛的烯丙基化
- 脱羧酶 C-C 键断裂反应
- 氢化铼/硼路易斯酸共催化烯烃加氢反应
- 不饱和肟醚的区域选择性羟基烷基化
N -杂环卡宾硼烷还原烷基溴的反应物
具有氧化电位的四甲基铵三烷基苯硼酸酯盐合成用反应物
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1A - STOT SE 3 - Water-react 2
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
商品
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持