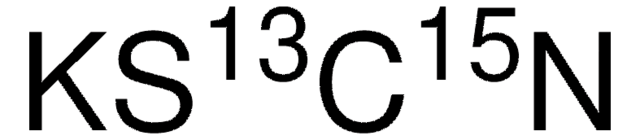推荐产品
化驗
97%
mp
112-116 °C (lit.)
官能基
bromo
SMILES 字串
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChI 密鑰
REFDOIWRJDGBHY-UHFFFAOYSA-N
應用
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
T J Monks et al.
Molecular pharmacology, 34(1), 15-22 (1988-07-01)
The formation of potentially reactive thiols has been postulated to play a role in the nephrotoxicity caused by a number of glutathione and/or cysteine conjugates. However, the inherent reactivity of such compounds has precluded both their identification in biological systems
T J Monks et al.
Drug metabolism and disposition: the biological fate of chemicals, 13(5), 553-559 (1985-09-01)
Incubation of either o-bromophenol or 2-bromohydroquinone with rat liver microsomes and 0.25 mM 35S-glutathione (GSH) gave rise to several isomeric 35S-GSH conjugates. A mixture of these isomeric GSH conjugates was prepared chemically and two were purified by HPLC; 1H-NMR spectroscopy
R G Schnellmann
Toxicology and applied pharmacology, 99(1), 11-18 (1989-06-01)
2-Bromohydroquinone (BHQ) plays an important role in bromobenzene-induced nephrotoxicity and is a model toxic hydroquinone. Since BHQ has a quinone nucleus and various quinones have been shown to produce cytotoxicity via oxidative stress, the goal of this study was to
G W Miller et al.
Toxicology and applied pharmacology, 125(2), 192-197 (1994-04-01)
We have previously shown that strychnine mimics the cytoprotective properties of glycine in renal proximal tubule (RPT) suspensions exposed to antimycin A (AA). The aims of this study were to determine whether the cytoprotective properties of strychnine applied to various
S S Lau et al.
Biochemical and biophysical research communications, 152(1), 223-230 (1988-04-15)
2-Bromo-(diglutathion-Syl)hydroquinone (2-Br-[diGSyl]HQ) is a more potent nephrotoxicant than any of three mono-substituted isomers. The reason for this differential toxicity is unknown. We now report that the rate of uptake of 2-Br-(diGSyl)HQ, 2-Br-3-(GSyl)HQ, 2-Br-5-(GSyl)-HQ and 2-Br-6(GSyl)HQ by kidney slices was 2.4
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门