所有图片(3)
About This Item
经验公式(希尔记法):
C9H19N2O
CAS号:
分子量:
171.26
Beilstein:
3933966
EC號碼:
MDL號碼:
分類程式碼代碼:
12352000
PubChem物質ID:
NACRES:
NA.22
推荐产品
應用
可用于研究生物系统、聚合物的自旋标记物,并且可作为更复杂的自旋标记物的结构单元。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
N Naber et al.
Biochemistry, 33(13), 3855-3861 (1994-04-05)
Each actin molecule contains a nucleotide, tightly bound in a deep cleft that divides the molecule. To probe conformational changes within this region of the molecule, we have incorporated two spin label analogues of ATP into actin. In both analogs
Journal of Colloid and Interface Science, 165, 236-236 (1994)
SEC-MALS analysis of cellouronic acid prepared from regenerated cellulose by TEMPO-mediated oxidation.
Shibata I, et al.
Cellulose, 13(1), 73-80 (2006)
Darja Jaušovec et al.
Carbohydrate polymers, 116, 74-85 (2014-12-03)
The chemo-enzymatic modification of cellulose nanofibers (CNFs) using laccase as biocatalysts and TEMPO or 4-Amino-TEMPO as mediators under mild aqueous conditions (pH 5, 30 °C) has been investigated to introduce surface active aldehyde groups. 4-Amino TEMPO turned out to be
Spin-Labeled Dendrimers in EPR Imaging with Low Molecular Weight Nitroxides.
Alexander T. Yordanov et al.
Angewandte Chemie (International ed. in English), 40(14), 2690-2692 (2001-07-18)
商品
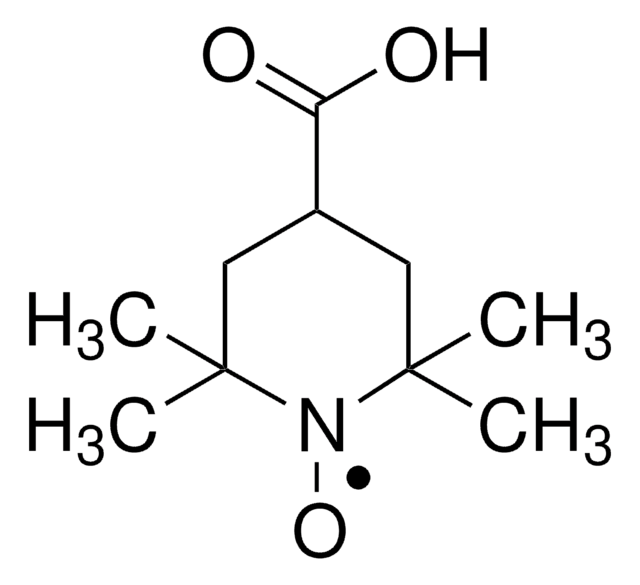
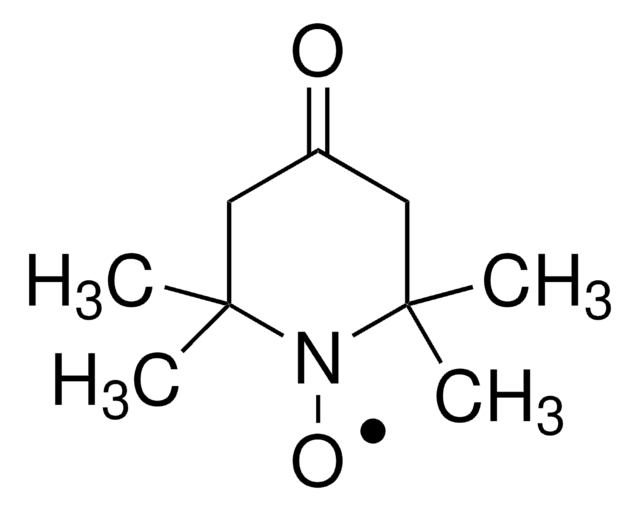
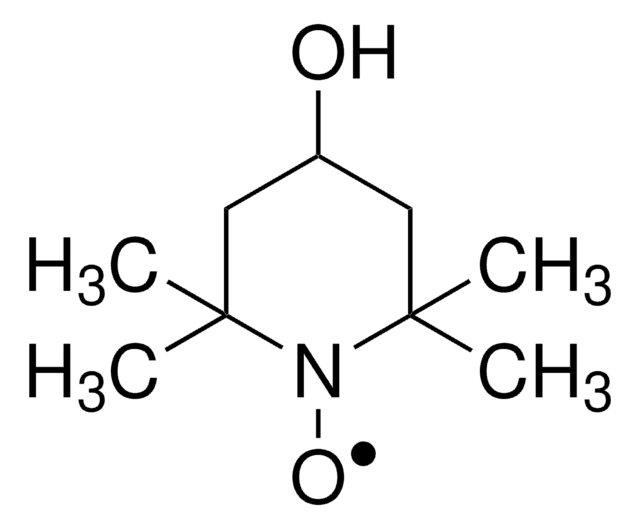
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持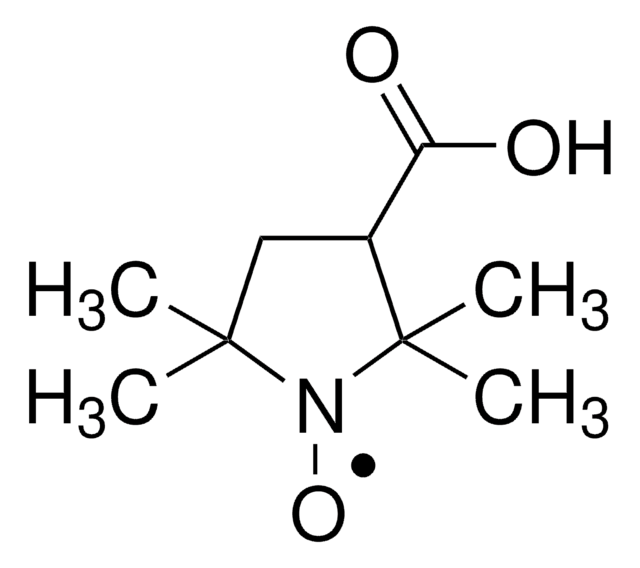

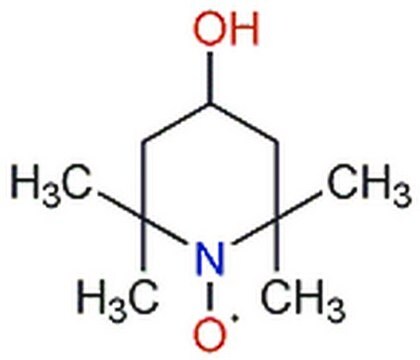
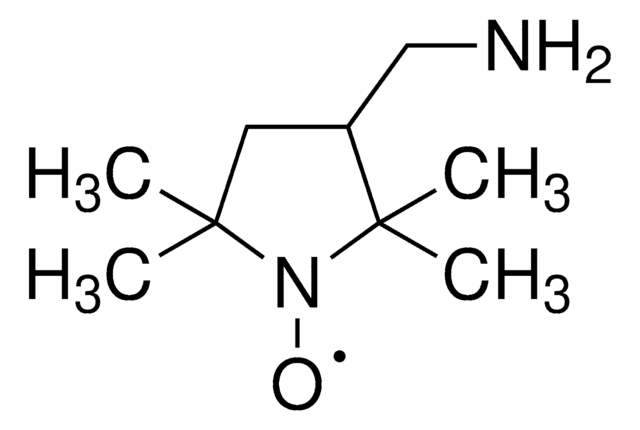
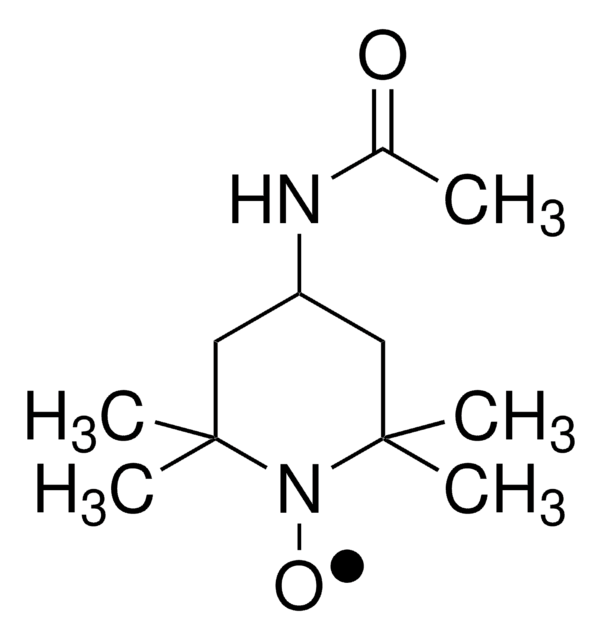
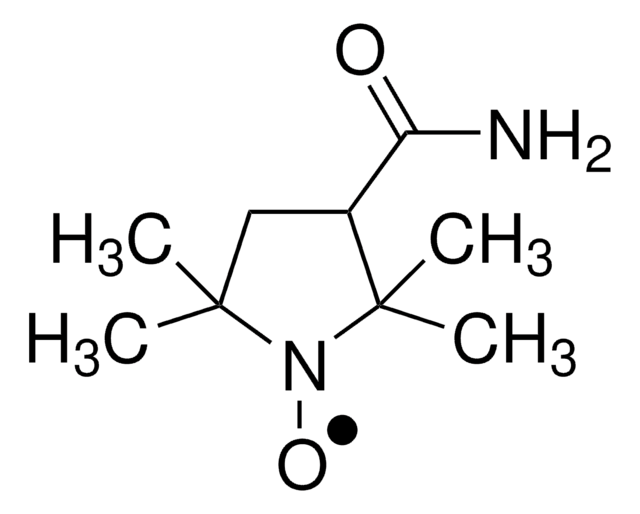

![2,2,6,6-四甲基-4- [1-氧代-6-(三乙铵)己氨基] -1-哌啶基氧基溴化物 95%](/deepweb/assets/sigmaaldrich/product/structures/398/827/e455c61a-b8fc-4800-9d17-107d734f6aa8/640/e455c61a-b8fc-4800-9d17-107d734f6aa8.png)