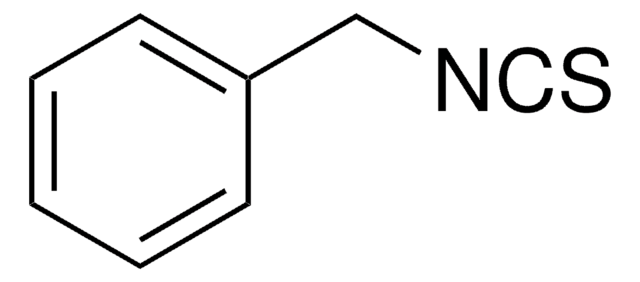
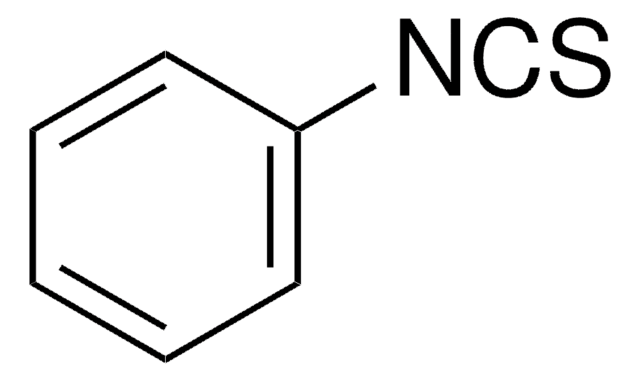
推荐产品
品質等級
化驗
≥95.0% (GC)
形狀
solid
bp
230-235 °C (lit.)
mp
39-41 °C (lit.)
39-41 °C
溶解度
diethyl ether: soluble 0.5 g/10 mL, clear to very faintly turbid, colorless to almost colorless
官能基
phenyl
thiocyanate
thioether
儲存溫度
2-8°C
SMILES 字串
N#CSCc1ccccc1
InChI
1S/C8H7NS/c9-7-10-6-8-4-2-1-3-5-8/h1-5H,6H2
InChI 密鑰
ABNDFSOIUFLJAH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Benzyl thiocyanate undergoes regioselective bond dissociation during its electrochemical reduction in acetonitrile at an inert electrode. It is added to stimulate the chlortetracycline biosynthesis during industrial fermentations. It undergoes biotransformation into dibenzyl disulphide by Streptomyces aureofaciens.
應用
Benzyl thiocyanate was used to study the effects of various dietary compounds on the α-hydroxylation of N-nitrosopyrrolidine in male F344 rats in vitro.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F
閃點(°C)
110 °C
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
S Sugie et al.
Carcinogenesis, 15(8), 1555-1560 (1994-08-01)
The effects of two aromatic thiocyanates, benzyl thiocyanate (BTC) and benzyl isothiocyanate (BITC), on methylazoxymethanol (MAM) acetate-induced intestinal carcinogenesis were examined using female ACI/N rats. Starting at 5 weeks of age, animals were fed diets containing 100 or 400 p.p.m.
F L Chung et al.
Cancer research, 44(7), 2924-2928 (1984-07-01)
Male F344 rats were pretreated with various dietary compounds, and the effects of pretreatment on the in vitro alpha-hydroxylation of N-nitrosopyrrolidine or N'-nitrosonornicotine were determined in assays with liver microsomes or cultured esophagus, respectively. Dietary compounds included phenols, cinnamic acids
Abdelaziz Houmam et al.
Journal of the American Chemical Society, 125(42), 12676-12677 (2003-10-16)
The electrochemical reduction of benzyl thiocyanate and p-nitrobenzyl thiocyanate was investigated in acetonitrile at an inert electrode. These two compounds reveal a change in the reductive cleavage mechanism, and more interestingly, they show a clear-cut example of a regioselective bond
J Fuska et al.
Letters in applied microbiology, 19(3), 124-125 (1994-09-01)
Benzyl thiocyanate, a specific stimulator of chlortetracycline biosynthesis, was transformed into dibenzyl disulphide by Streptomyces aureofaciens. The disulphide stimulated chlortetracycline production to a lesser extent than did benzyl thiocyanate.
X M Li et al.
Applied microbiology and biotechnology, 57(5-6), 717-724 (2002-01-10)
Changes in synthesis and abundance of proteins associated with chlortetracycline (CTC) production in Streptomyces aureofaciens were investigated by two-dimensional polyacrylamide gel electrophoresis of proteins pulse-labelled in vivo with L-[35S]methionine. Eleven individual protein spots were selected as being related to formation
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门