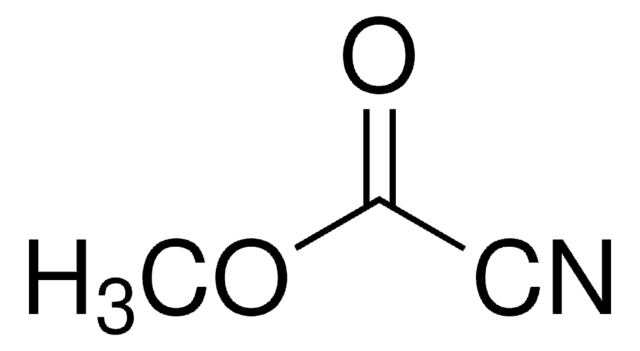
推荐产品
化驗
98%
形狀
solid
bp
206 °C (lit.)
mp
28-31 °C (lit.)
密度
1.106 g/mL at 25 °C (lit.)
官能基
ketone
nitrile
phenyl
SMILES 字串
O=C(C#N)c1ccccc1
InChI
1S/C8H5NO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H
InChI 密鑰
GJQBHOAJJGIPRH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
用苯甲酰氰化物研究苯甲酰氰化物在乙腈、N,N-二甲基甲酰胺和乙腈中的还原机理 。它经过 红球菌 CCZU10-1水解形成苯甲酰甲酸 。
應用
氨基化合物的选择性酰化反应试剂。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral
儲存類別代碼
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
183.2 °F - closed cup
閃點(°C)
84 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Synthesis, 433-433 (1993)
Weisi Xiong et al.
Biotechnology letters, 37(8), 1703-1709 (2015-04-22)
To develop a biphasic system for use in plant tissue-mediated biotransformations to overcome the low water solubilities of substrates and inhibitory effects of products. Commonly-used organic solvents and ionic liquid were assayed using the biphasic system to reduce water-insoluble benzoyl
Yi Xu et al.
Journal of virology, 92(11) (2018-03-09)
Translational readthrough of the stop codon of the capsid protein (CP) open reading frame (ORF) is used by members of the Luteoviridae to produce their minor capsid protein as a readthrough protein (RTP). The elements regulating RTP expression are not
Petar I Penev et al.
Genome biology and evolution, 12(10), 1694-1710 (2020-08-14)
The ribosome's common core, comprised of ribosomal RNA (rRNA) and universal ribosomal proteins, connects all life back to a common ancestor and serves as a window to relationships among organisms. The rRNA of the common core is similar to rRNA
Norma A Macías-Ruvalcaba et al.
The Journal of organic chemistry, 72(2), 589-594 (2007-01-16)
The mechanism of reduction of benzoyl cyanide, 6, p-methoxybenzoyl cyanide, 7, and p-chlorobenzoyl cyanide, 8, has been studied in acetonitrile (6 and 7), N,N-dimethylformamide (6), and acetonitrile containing water (all three compounds). The reaction proceeds by initial reduction to form
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门