推荐产品
等級
produced by Wacker Chemie AG, Burghausen, Germany
化驗
≥96.0% (GC)
包含
~0.1% Drapex 39 as stabilizer
製造商/商標名
Wacker Chemie AG
折射率
n20/D 1.432 (lit.)
bp
120 °C (lit.)
溶解度
H2O: soluble 10 parts
alcohol: miscible
chloroform: miscible
diethyl ether: miscible
密度
1.162 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
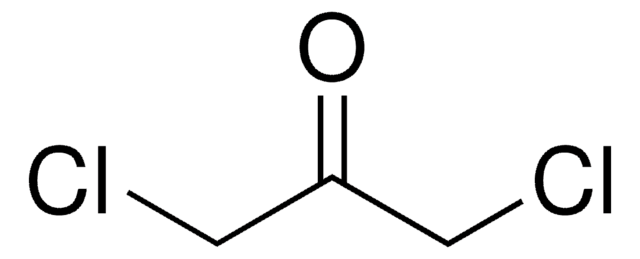
SMILES 字串
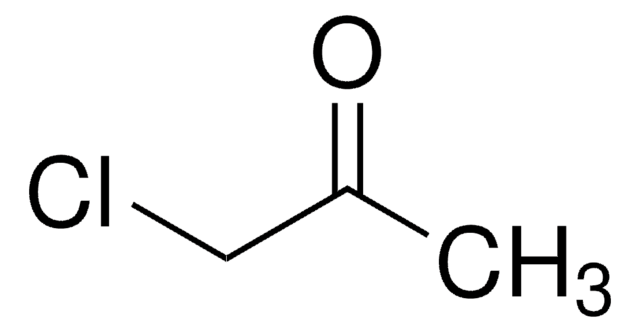
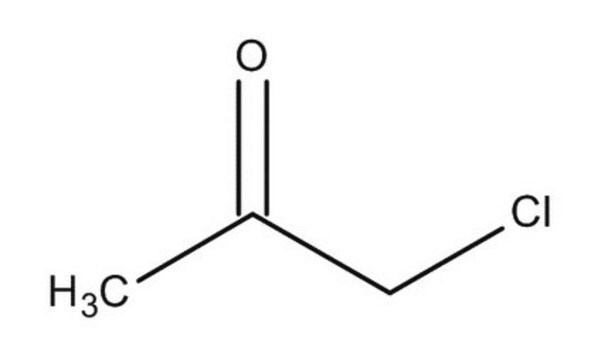
CC(=O)CCl
InChI
1S/C3H5ClO/c1-3(5)2-4/h2H2,1H3
InChI 密鑰
BULLHNJGPPOUOX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
氯丙酮与L-脯氨酸酰胺催化的 4-硝基苯甲醛发生直接羟醛反应。它与羧酸反应形成酯衍生物。
應用
氯丙酮用于氯丙酮的微生物不对称还原合成手性 1,2-环氧丙烷。它用于通过气相色谱分析酸。它还用于和 1-((3-(萘并[2,1-b]呋喃-2-基)-1-苯基-1H-吡唑-4-基)亚甲基)-2-苯腙反应合成甲基噻唑衍生物。
其他說明
可根据要求提供批量价格
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Analysis of carboxylic acids by gas chromatography. Derivatisation using chloroacetone.
McCalley DV, et al.
Chromatographia, 18(6), 309-312 (1984)
Synthesis of optically pure 1, 2-epoxypropane by microbial asymmetric reduction of chloroacetone.
Weijers CAGM, et al.
Applied Microbiology and Biotechnology, 38(3), 297-300 (1992)
Ashraf H F Abd El-Wahab et al.
Molecules (Basel, Switzerland), 16(1), 307-318 (2011-01-27)
Vilsmeier formylation of 2-(1-phenylhydrazonoethyl)naphtho[2,1-b]furan (2) gave 3-naphtho[2,1-b]furan-2-yl-1-phenyl-1H-pyrazole-4-carbaldehyde (3), which was reacted with C- and N-nucleophiles to afford naphthofuranpyrazol derivatives 4-8. Treatment of 2-[(3-(naphtho[2,1-b]furan-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene]-malononitrile (4a) with reactants having active hydrogen and Et₃N gave the corresponding pyrazoline, pyran and chromene addition product
L-Proline amide-catalyzed direct asymmetric aldol reaction of aldehydes with chloroacetone.
He L, et al.
Tetrahedron, 62(2), 346-351 (2006)
Dose-response relationships for mutations induced in E. coli by some model compounds. With an addendum: Reaction kinetics in water of chloroethylene oxide, chloroacetaldehyde, and chloroacetone.
S Hussain et al.
Hereditas, 101(1), 57-68 (1984-01-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门