MS0100
ProteoMass™ Guanidination Kit
For improving MALDI-MS sensitivity and peptide sequence coverage
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.24
Recommended Products
Quality Level
product line
ProteoMass™
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
compatibility
for use with (Complex cell extracts, pure protein solution, 1D and 2D PAGE gels)
Application
The ProteoMass Guanidination Kit allows you to enhance MALDI-MS sensitivity, increase sequence coverage, and identify peptides with greater confidence. Following proteolytic digestion, peptides with C-terminal Arginine residues are ionized preferentially over peptides with C-terminal Lysine residues, leading to compromised sequence coverage and limited confidence during peptide mass fingerprint analysis. The ProteoMass Guanidination Kit efficiently and conveniently converts C-terminal Lysine residues to homoarginine, increasing MALDI signal strength and producing enhanced sequence coverage.
Features and Benefits
- Identify more samples with greater accuracy and confidence
- Increase throughput and save time - only 35 minutes to use the kit vs. 2 hours using traditional methods
- Compatibility - compatible with 1D or 2D PAGE gel bands or spots, as well as complex cell extracts
Other Notes
One MS0100 kit is sufficient for 96 reactions.
Legal Information
ProteoMass is a trademark of Sigma-Aldrich Co. LLC
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yoshimi Kanie et al.
PloS one, 4(5), e5434-e5434 (2009-05-06)
A variety of N-glycans attached to protein are known to involve in many important biological functions. Endoplasmic reticulum (ER) and Golgi localized enzymes are responsible to this template-independent glycan synthesis resulting glycoforms at each asparagine residues. The regulation mechanism such
Fumihiro Oshita et al.
Oncology reports, 24(3), 637-645 (2010-07-29)
To investigate the early changes in protein function that induce micro-metastasis in early-stage non-small cell lung cancer, we conducted proteomic analysis of tissue that had been completely resected. We selected sixteen patients whose tumors were pathological stage I adenocarcinoma with
Roberta Cozzi et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(5), 2008-2018 (2012-01-19)
Group B Streptococcus pili are covalently linked structures assembled via a sortase-catalyzed transpeptidation mechanism involving specific residues and motifs. A sequence element containing a conserved glutamic acid, called the E-box, has been described to be involved in pilus formation. Although
Alberto Nuñez et al.
Journal of agricultural and food chemistry, 57(22), 10951-10958 (2009-10-29)
Several studies have suggested that the emulsification properties associated with pectin obtained from sugar beet (Beta vulgaris) are due to the presence of a protein-pectin complex. Nevertheless, the identity of the protein has remained elusive. Pectin, extracted from sugar beet
Joungil Choi et al.
The Journal of biological chemistry, 281(16), 10816-10824 (2006-03-07)
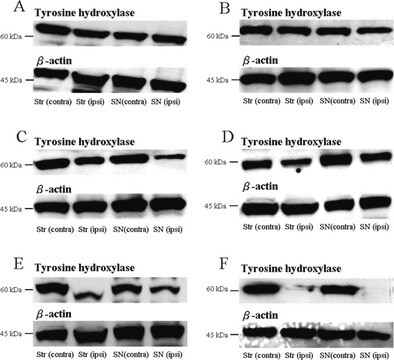
Mutations in DJ-1 cause an autosomal recessive, early onset familial form of Parkinson disease (PD). However, little is presently known about the role of DJ-1 in the more common sporadic form of PD and in other age-related neurodegenerative diseases, such
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service