389579
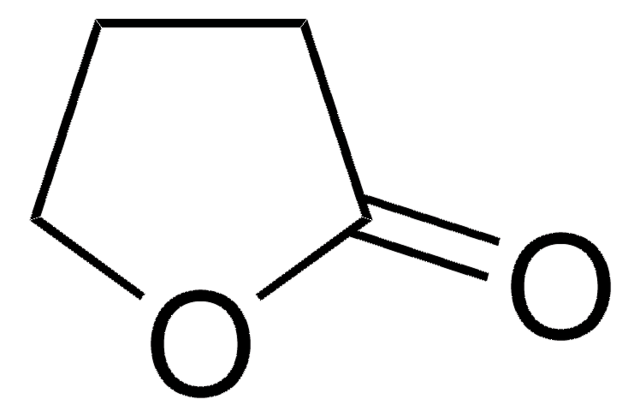
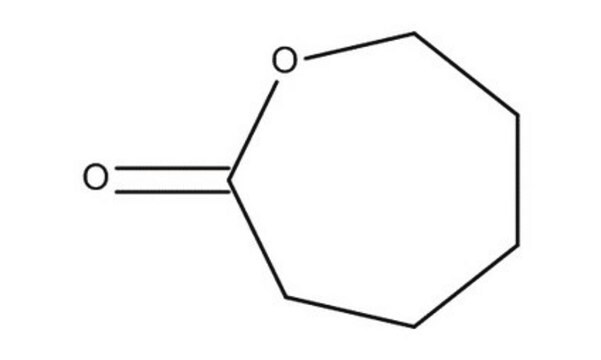
δ-Valerolactone
technical grade
Synonym(s):
delta-Valerolactone, Tetrahydro-2H-2-pyranone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein:
106436
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
liquid
impurities
<25% polymer
refractive index
n20/D 1.457 (lit.)
bp
226-229 °C (lit.)
58-60 °C/0.5 mmHg (lit.)
mp
−13-−12 °C (lit.)
density
1.079 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
O=C1CCCCO1
InChI
1S/C5H8O2/c6-5-3-1-2-4-7-5/h1-4H2
InChI key
OZJPLYNZGCXSJM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
δ-Valerolactone (tetrahydro-2H-2-pyranone or δ VL) can be used as a monomer unit in the synthesis of poly(δ-valerolactone)s poly(conjugated ester)s via ring-opening polymerization.
It can also be used as a starting material in the synthesis of (+)-guadinomic acid , sodium δ-hydroxyvalerate , methyl δ-hydroxyvalerate , and 5-hydroxyvaleraldehyde.
It can also be used as a starting material in the synthesis of (+)-guadinomic acid , sodium δ-hydroxyvalerate , methyl δ-hydroxyvalerate , and 5-hydroxyvaleraldehyde.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ryo Shintani et al.
Organic letters, 14(9), 2410-2413 (2012-04-26)
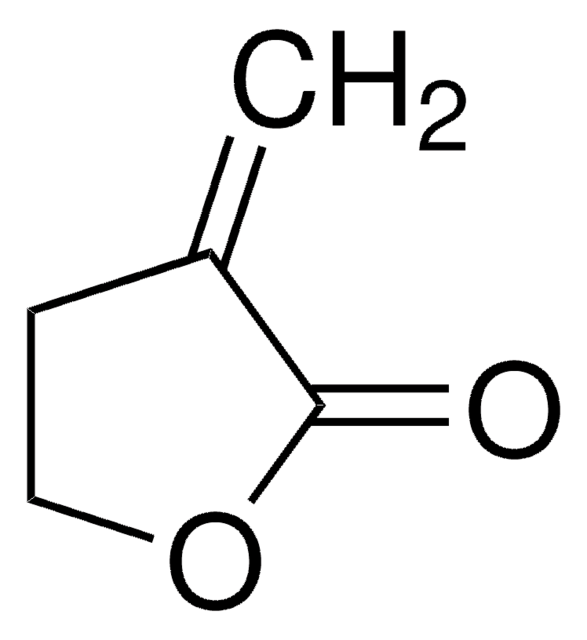
A palladium-catalyzed decarboxylative cyclopropanation of γ-methylidene-δ-valerolactones with aromatic aldehydes has been developed to give 4-oxaspiro[2.4]heptanes with high selectivity. The site of nucleophilic attack to a π-allylpalladium intermediate has been controlled with a sterically demanding phosphine ligand. The course of the
Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone
Makiguchi K, et al.
Macromolecules, 44(7), 1999-2005 (2011)
K N Houk et al.
The Journal of organic chemistry, 73(7), 2674-2678 (2008-03-08)
gamma-Butyrolactone, unlike delta-valerolactone, does not polymerize despite a strain energy of approximately 8 kcal mol-1 which could be relieved by opening the s-cis lactone ester bond to an s-trans ester bond in the polymer. To explain this anomaly, we have
Organolanthanide-initiated living polymerizations of ε -caprolactone, δ-valerolactone, and β-propiolactone
Yamashita M, et al.
Macromolecules, 29(5), 1798-1806 (1996)
Zuwei Ma et al.
Biomacromolecules, 12(9), 3265-3274 (2011-07-16)
Biodegradable polyurethane urea (PUU) elastomers are ideal candidates for fabricating tissue engineering scaffolds with mechanical properties akin to strong and resilient soft tissues. PUU with a crystalline poly(ε-caprolactone) (PCL) macrodiol soft segment (SS) showed good elasticity and resilience at small
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service