202215
Sodium tetrafluoroborate
98%
Synonym(s):
Sodium fluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NaBF4
CAS Number:
Molecular Weight:
109.79
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
Assay:
98%
form:
powder or crystals
solubility:
water: soluble(lit.)
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
solubility
water: soluble(lit.)
density
2.47 g/mL at 25 °C (lit.)
SMILES string
[Na+].F[B-](F)(F)F
InChI
1S/BF4.Na/c2-1(3,4)5;/q-1;+1
InChI key
KGJZTOFHXCFQIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sodium tetrafluoroborate (NaBF4) is colorless sodium salt and its crystals belong to the rhombic crystal system. It can be synthesized by reacting tetrafluoroboric acid with sodium carbonate or hydroxide.
Application
Sodium tetrafluoroborate may be used as a catalyst for the synthesis of bis(indolyl)methanes via electrophilic substitution reaction of indoles with aldehydes and ketones. It may also be used as a source of tetrafluoroborate anoins during the synthesis of ionic liquids like 1-butyl-3-methylimidazolium tetrafluoroborate and trihexyl(tetradecyl)phosphonium tetrafluoroborate.
Sodium tetrafluoroborate may be used for the laboratory synthesis of boron fluoride. It may be used for the synthesis of fluoro-nucleic acids and fluoroheterocyclic compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Physical and electrochemical properties of 1-butyl-3-methylimidazolium bromide, 1-butyl-3-methylimidazolium iodide, and 1-butyl-3-methylimidazolium tetrafluoroborate.
Kim KS, et al.
Korean Journal of Chemical Engineering, 21(5), 1010-1014 (2004)
Highly efficient synthesis of bis (indolyl) methanes catalyzed by sodium tetrafluoroborate.
Kamble VT, et al.
Chin. J. Chem., 25(1), 13-15 (2007)
Industrial preparation of phosphonium ionic liquids.
Bradaric CJ, et al.
Green Chemistry, 5(2), 143-152 (2003)
Facile direct conversion of amino-heterocycles to fluoro-heterocycles using t-butylthionitrite or t-butylthionitrate with sodium tetrafluoroborate.
Yong HK, et al.
Tetrahedron Letters, 31(21), 3019-3022 (1990)
Eagleson M.
Concise Encyclopedia Chemistry, 1001-1001 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

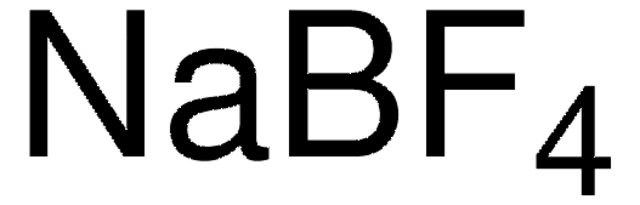






![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)