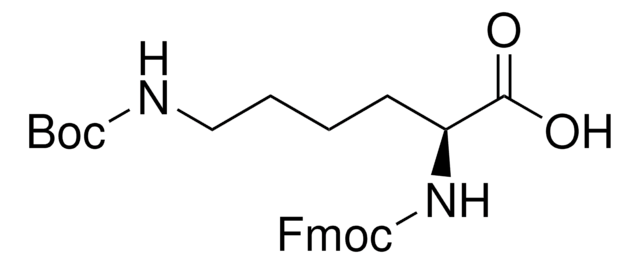
04936
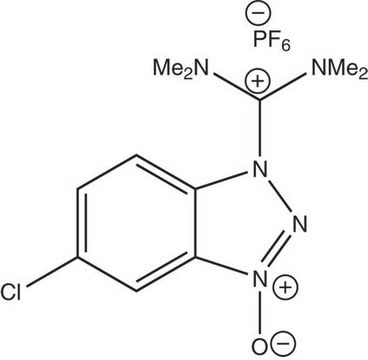
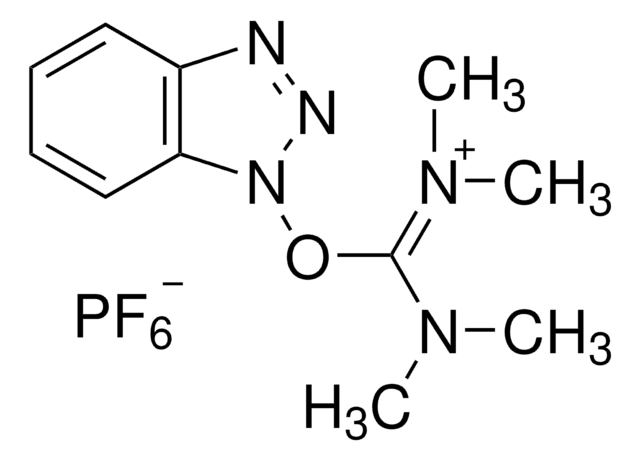
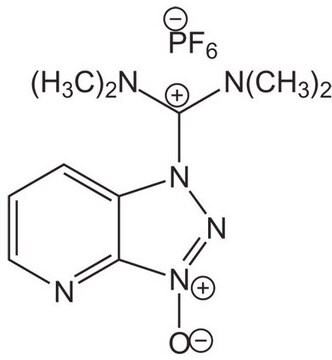
O-(6-Chlorobenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
≥98.0% (HPLC)
Synonym(s):
1-[Bis(dimethylamino)methylen]-5-chlorobenzotriazolium 3-oxide hexafluorophosphate, N,N,N′,N′-Tetramethyl-O-(6-chloro-1H-benzotriazol-1-yl)uronium hexafluorophosphate, HCTU
About This Item
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: Coupling Reactions
impurities
≤0.5% water
mp
185-190 °C
application(s)
peptide synthesis
functional group
amine
chloro
SMILES string
ClC1=CC=C(N([C+](N(C)C)N(C)C)N=[N+]2[O-])C2=C1.F[P-](F)(F)(F)(F)F
InChI
1S/C11H15ClN5O.F6P/c1-15(2)11(16(3)4)18-17-10-7-8(12)5-6-9(10)13-14-17;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1
InChI key
ZHHGTMQHUWDEJF-UHFFFAOYSA-N
Application
Synthesis of near-infrared pH activatable fluorescent probes
Synthesis of human β-amyloid by Fmoc chemistry
Stereoselective Horner-Wadsworth-Emmons olefination
Covalent ligation of fluorescent peptides to quantum dots
Alkylation of human telomere sequence
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service