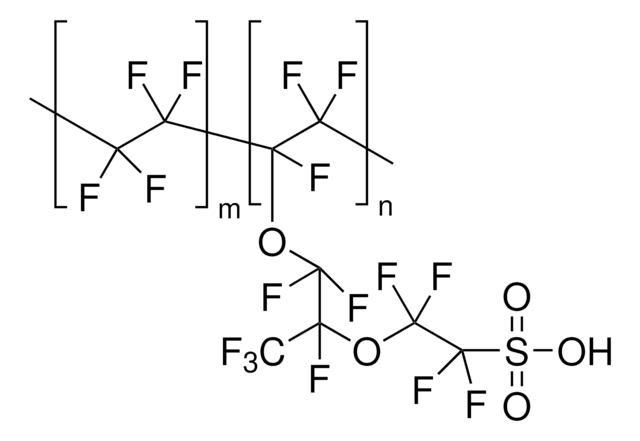
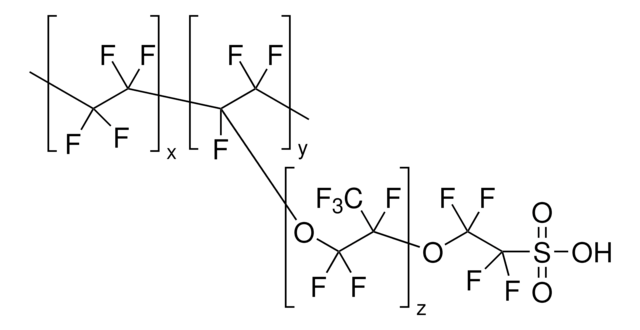
510211
Nafion™ perfluorinated resin solution
5 wt. % in mixture of lower aliphatic alcohols and water, contains 45% water
Synonym(s):
D-521
About This Item
Recommended Products
eq. wt.
1,100
contains
45% water
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
5 wt. % in mixture of lower aliphatic alcohols and water
refractive index
n20/D 1.368
bp
84 °C
density
0.921 g/mL at 25 °C
greener alternative category
InChI
1S/C7HF13O5S.C2F4/c8-1(9)2(10)24-5(15,16)3(11,4(12,13)14)25-6(17,18)7(19,20)26(21,22)23;3-1(4)2(5)6/h(H,21,22,23);
InChI key
FOYUGSIADQEOEK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Physical form
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - STOT SE 2 - STOT SE 3
Target Organs
Eyes,Central nervous system, Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Materials Issues in Polymer Electrolyte Membrane Fuel Cells
Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures and are composed of two electrodes and a conductive elecrolyte.
Advances in the electrochemical conversion of water to and from hydrogen and oxygen have principally been achieved through the development of new materials and by understanding the mechanisms of the degradation of proton exchange membrane fuel cells (PEMFC) during operation.
Related Content
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service