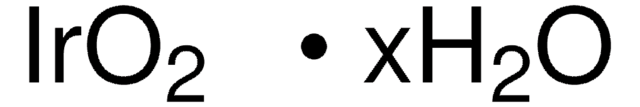208833
Ruthenium(IV) oxide hydrate
powder
Synonym(s):
Hydrous ruthenium oxide, Ruthenium dioxide hydrate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
RuO2 · xH2O
CAS Number:
Molecular Weight:
133.07 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
reaction suitability
reagent type: catalyst
core: ruthenium
SMILES string
O.O=[Ru]=O
InChI
1S/H2O.2O.Ru/h1H2;;;
InChI key
FGEKTVAHFDQHBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ramin M A Tehrani et al.
Biosensors & bioelectronics, 38(1), 278-283 (2012-06-30)
A non-enzymatic glucose sensor of multi-walled carbon nanotube-ruthenium oxide/composite paste electrode (MWCNT-RuO(2)/CPE) was developed. The electrode was characterized by using XRD, SEM, TEM and EIS. Meanwhile, cyclic voltammetry and amperometry were used to check on the performances of the MWCNT-RuO(2)/CPE
Guolei Xiang et al.
Scientific reports, 2, 801-801 (2012-11-13)
Controls over the atomic dispersity and particle shape of noble metal catalysts are the major qualities determining their usability in industrial runs, but they are usually difficult to be simultaneously realized. Inspired from the Deacon catalyst in which RuO(2) can
Todd P Luxton et al.
Journal of colloid and interface science, 359(1), 30-39 (2011-04-23)
Ruthenium oxides (RuO(2)·1·10H(2)O and RuO(2)) have been synthesized by forced hydrolysis and oxidation of ruthenium chloride. The resulting materials were extensively characterized to determine the crystallinity, surface area, and ruthenium oxidation state. Surface charging experiments indicate a large quantity of
Jelena Radjenovic et al.
Water research, 45(4), 1579-1586 (2010-12-21)
During membrane treatment of secondary effluent from wastewater treatment plants, a reverse osmosis concentrate (ROC) containing trace organic contaminants is generated. As the latter are of concern, effective and economic treatment methods are required. Here, we investigated electrochemical oxidation of
Dong-Jin Yun et al.
ACS applied materials & interfaces, 4(9), 4588-4594 (2012-08-23)
RuO(2) films were deposited on SiO(2) (300 nm)/N++Si substrates using radio frequency magnetron sputtering at room temperature. As-deposited RuO(2) films were annealed at different temperatures (100, 300, and 500 °C) and ambients (Ar, O(2) and vacuum), and the resulting effects
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service