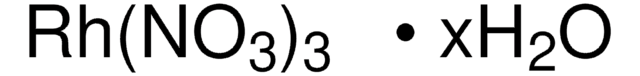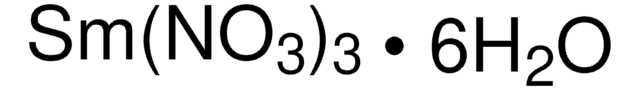373567
Ruthenium(III) nitrosyl nitrate solution
in dilute nitric acid
Synonym(s):
Nitrilooxonium ruthenium(2+) nitrate (1:1:3)
About This Item
Recommended Products
form
liquid
composition
Ru, 1.5% (typical)
reaction suitability
reagent type: catalyst
core: ruthenium
concentration
in dilute nitric acid
density
1.07 g/mL at 25 °C
SMILES string
[Ru+3]N=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/3NO3.NO.Ru/c3*2-1(3)4;1-2;/q4*-1;+4
InChI key
VAAILMLOAHTPQK-UHFFFAOYSA-N
General description
Application
- As a Ru source to prepare Y2Ru2O7/NiMoO4@NF electrocatalyst by a combined sol–gel and hydrothermal method, which exhibits superior stability for overall water splitting in an alkaline medium.
- To fabricate bifunctional electrode for Li-air batteries.
- As a precursor to synthesize ruthenium nanoparticles with variable sizes. It helps to form stable ruthenium nitrite complex avoiding the unwanted metal oxide hydrolysis or precipitation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Hydrogen is one of the most important resources in providing food, fuel, and chemical products for our everyday life. Sustainable catalytic hydrogen production from bioethanol has gained significant attention in recent years due to globally diminishing fossil fuel supplies, which have necessitated the search for new chemical feedstocks.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service