1708707
USP
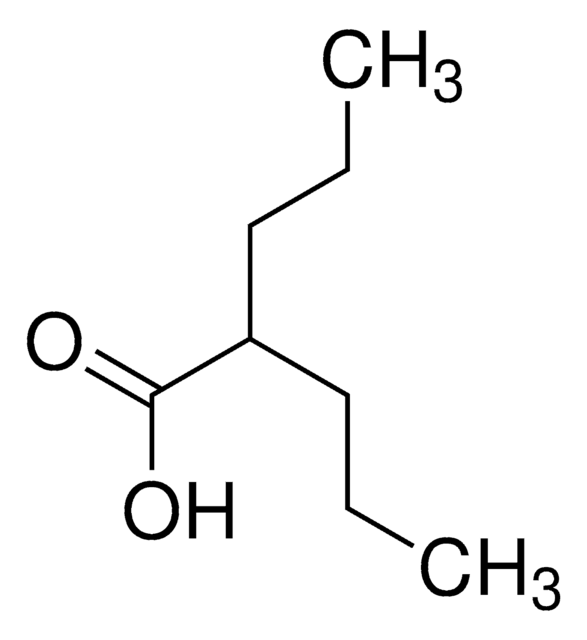
Valproic acid
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
2-Propylpentanoic acid, Valproic acid
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
valproic acid
Fabricant/nom de marque
USP
Indice de réfraction
n20/D 1.425 (lit.)
Point d'ébullition
220 °C (lit.)
Densité
0.9 g/mL at 25 °C (lit.)
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
CCCC(C(O)=O)CCC
InChI
1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
Clé InChI
NIJJYAXOARWZEE-UHFFFAOYSA-N
Informations sur le gène
human ... ALDH5A1(7915)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Remarque sur l'analyse
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 1A - Skin Irrit. 2
Code de la classe de stockage
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
231.8 °F - closed cup
Point d'éclair (°C)
111 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique