T2859
Tamoxifène
powder, suitable for cell culture, BioReagent
Synonyme(s) :
(Z)-1-(p-Diméthylaminoéthoxyphényl)-1,2-diphényl-1-butène, (Z)-2-[4-(1,2-Diphénylbut-1-ényl)phénoxy]-N,N-diméthyléthanamine
About This Item
Produits recommandés
product name
Tamoxifène, powder, Suitable for cell culture
Niveau de qualité
Pureté
≥99%
Forme
powder
Conditions de stockage
protect from light
Pf
97-98 °C (lit.)
Solubilité
H2O: insoluble <0.1% at 20 °C
chloroform: soluble 50 mg/mL, clear, colorless to faintly yellow
2-propanol: soluble
DMSO: soluble
ethanol: soluble
methanol: soluble
propylene glycol: soluble
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
cell membrane | interferes
enzyme | inhibits
Conditions d'expédition
ambient
Température de stockage
2-8°C
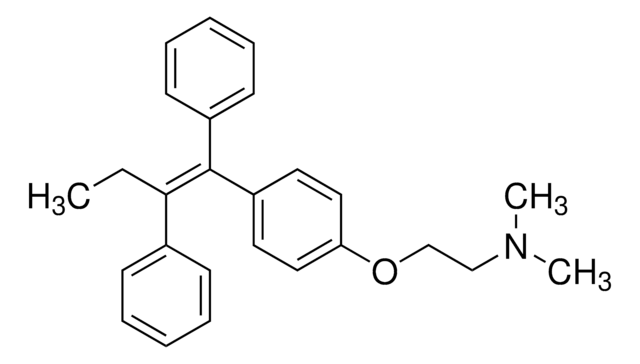
Chaîne SMILES
CC\C(c1ccccc1)=C(/c2ccccc2)c3ccc(OCCN(C)C)cc3
InChI
1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25-
Clé InChI
NKANXQFJJICGDU-QPLCGJKRSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
As an antibiotic, TAM increases membrane permeabilityand is active against Gram-positive and Gram- negative bacterial strains. It actsas both bactericidal and bacteriostatic agent.
Notes préparatoires
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Repr. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique