SRP0291
Cathepsin L Active human
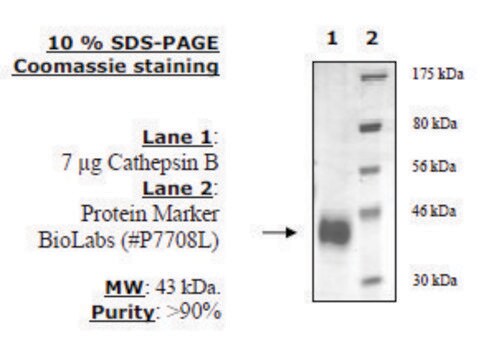
recombinant, expressed in FreeStyle™ 293-F cells, ≥90% (SDS-PAGE)
Synonyme(s) :
CATL, Major excreted protein (MEP)
About This Item
Produits recommandés
Source biologique
human
Produit recombinant
expressed in FreeStyle™ 293-F cells
Essai
≥90% (SDS-PAGE)
Forme
aqueous solution
Activité spécifique
≥3900 pmol/min-μg
Poids mol.
36 kDa
Technique(s)
inhibition assay: suitable
Adéquation
suitable for molecular biology
Numéro d'accès NCBI
Application(s)
life science and biopharma
Conditions d'expédition
dry ice
Température de stockage
−70°C
Informations sur le gène
human ... CTSL(1514)
Description générale
Cathepsin L is a papain-like cysteine protease and belongs to the Clan A, Family C1. It is composed of L domain of α-helix and an R domain of β-sheet in the spatial structure.
Human cathepsin L (GenBank Accession No. NM_001912), amino acids 18-333, with C-terminal HIS tag, MW = 36 kDa, expressed in FreeStyle 293-F cells.
Application
- to determine that N-acyl and N-sulfonyloxazolidine-2,4-diones are pseudo-irreversible inhibitors of serine proteases
- to investigate the role of cathepsin B and L activity in the serum during the human aging process.
- in inhibitory activity assay
- to study the role of CTSL in COVID-19 infection
Actions biochimiques/physiologiques
Définition de l'unité
Forme physique
Notes préparatoires
Informations légales
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique