L1254
L-Lactic Dehydrogenase from rabbit muscle
Type XI, lyophilized powder, 600-1,200 units/mg protein
Synonyme(s) :
Anaerobic Lactate Dehydrogenase, Lactate, NAD-lactate dehydrogenase, (S)-Lactate: NAD+ oxidoreductase, L-LDH, LAD, LD
About This Item
Produits recommandés
Source biologique
rabbit muscle
Type
Type XI
Forme
lyophilized powder
Activité spécifique
600-1,200 units/mg protein
Poids mol.
140 kDa
Composition
protein, 90-100%
Conditions de stockage
(Keep container tightly closed in a dry and well-ventilated place)
Technique(s)
activity assay: suitable
Couleur
white
Activité étrangère
pyruvate kinase, myokinase, malic dehydrogenase, glutamic-pyruvic transaminase, glutamic-oxalacetic transaminase and α-glycerophosphate dehydrogenase ≤0.01%
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
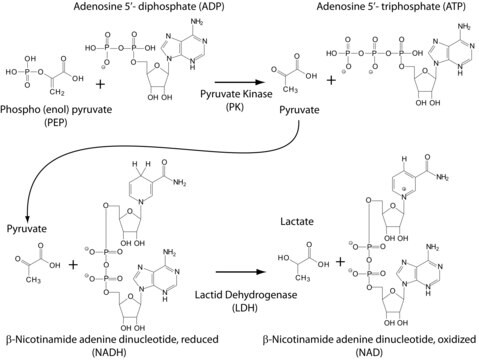
Description générale
Isoelectric point: 8.4-8.6
Optimal pH : 7.5 .
Application
- as a component of activation and relaxing solution in ATPase activity and isometric steady-state tension measurements with muscle fiber
- in Trypanosoma congolense pyruvate kinase activity assay
- in pyruvate kinase (PK) assay with rice plastidic PK enzyme OsPK2
Actions biochimiques/physiologiques
Définition de l'unité
Remarque sur l'analyse
Anticorps
Enzyme
Inhibiteur
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
For use as a marker in SDS-PAGE; lactate dehydrogenase; contaminant; L-Lactic Dehydrogenase from rabbit muscle, Type XI, lyophilized powder, 600-1,200 units/mg protein
For use as a marker in SDS-PAGE; Albumin from chicken egg white, For use as a marker in SDS-PAGE; L-Lactic Dehydrogenase from rabbit muscle, Type XI, lyophilized powder, 600-1,200 units/mg protein
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique