H6132
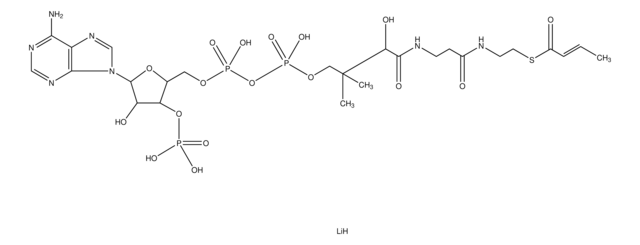
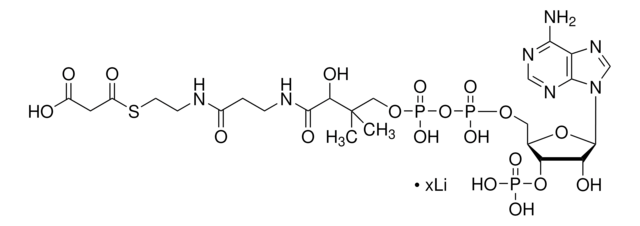
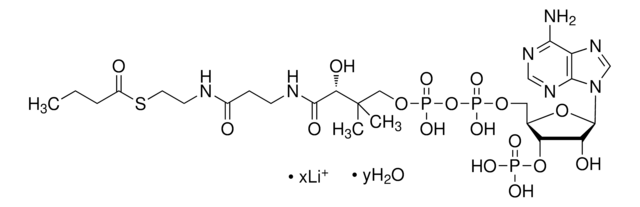
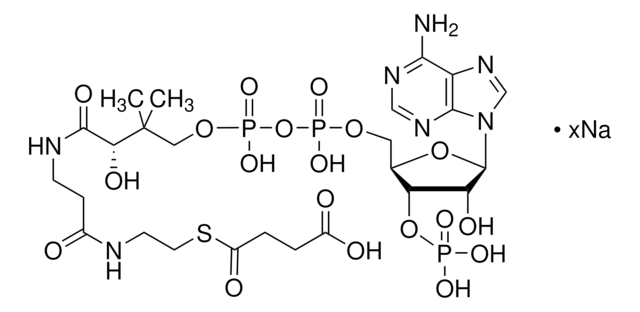
DL-3-Hydroxy-3-methylglutaryl coenzyme A sodium salt hydrate
≥90% (HPLC)
Synonyme(s) :
HMG-CoA
About This Item
Produits recommandés
Niveau de qualité
Essai
≥90% (HPLC)
Forme
powder
Solubilité
H2O: soluble-50 mg/mL, clear, colorless to faintly yellow
Température de stockage
−20°C
Chaîne SMILES
O.[Na+].[Na+].CC(O)(CC(O)=O)CC(=O)SCCNC(=O)CCNC(=O)[C@@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n2cnc3c(N)ncnc23
InChI
1S/C27H44N7O20P3S.2Na.H2O/c1-26(2,21(40)24(41)30-5-4-15(35)29-6-7-58-17(38)9-27(3,42)8-16(36)37)11-51-57(48,49)54-56(46,47)50-10-14-20(53-55(43,44)45)19(39)25(52-14)34-13-33-18-22(28)31-12-32-23(18)34;;;/h12-14,19-21,25,39-40,42H,4-11H2,1-3H3,(H,29,35)(H,30,41)(H,36,37)(H,46,47)(H,48,49)(H2,28,31,32)(H2,43,44,45);;;1H2/q;2*+1;/p-2/t14-,19-,20-,21-,25-,27?;;;/m1.../s1
Clé InChI
QGWMCHPRNDWWMT-UAUHQYJPSA-L
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
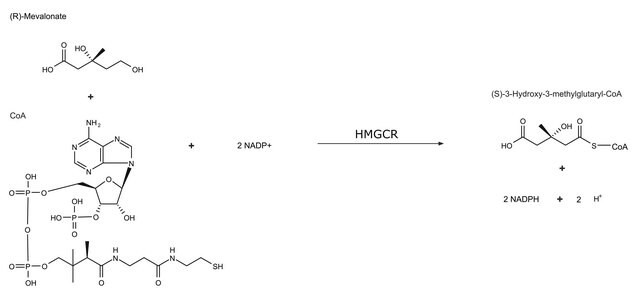
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
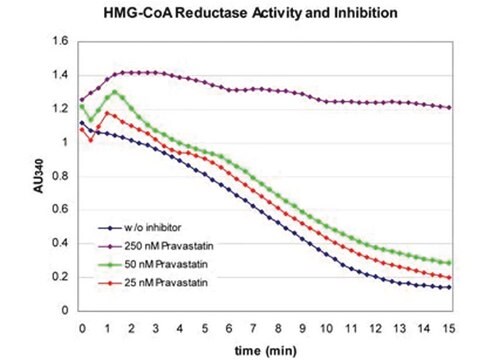
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique