F0162
Fibronectine
lyophilized powder, 45 kDa
Synonyme(s) :
Fibronectin
About This Item
Produits recommandés
Source biologique
human plasma
Niveau de qualité
Pureté
≥90% (SDS-PAGE)
Forme
lyophilized powder
Poids mol.
45 kDa
Conditionnement
pkg of 0.5 mg
Technique(s)
cell culture | mammalian: suitable
Impuretés
HIV and HBsAg, source material tested negative
Small proteolytic fragments, may contain traces
Solubilité
water: soluble ≥0.500 mg/mL, clear to slightly hazy, colorless
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Température de stockage
−20°C
Informations sur le gène
human ... FN1(2335)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
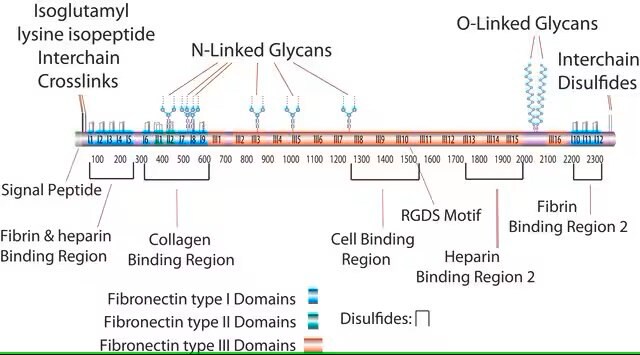
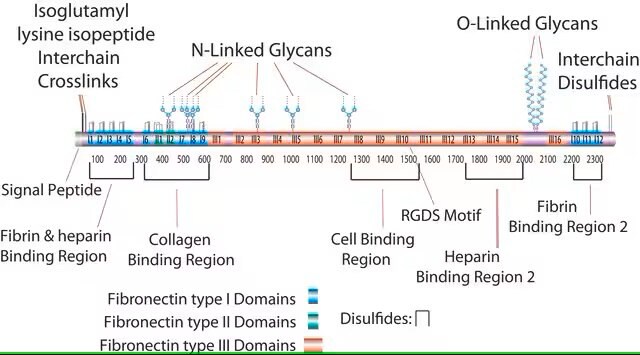
This fragment has an acidic pI (4.9-5.3) and does not bind to heparin. This domain is resistant to proteolysis due to intrachain disulfide bonding and the attached carbohydrate. The intrachain disulfide bonds are essential for binding to gelatin, while the complex, branched, asparagine-linked carbohydrate is not. This fragment binds to C1q, but not to fibrin.
Attention
Notes préparatoires
En option
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Extracellular matrix proteins such as laminin, collagen, and fibronectin can be used as cell attachment substrates in cell culture.
Protocoles
Dilute fibronectin to the desired concentration. Optimum conditions for attachment are dependent on cell type and application. The typical coating concentration is 1 – 5 ug/cm2.Fibronectin coating protocol, products, and FAQs at sigmaaldrich.com
Dilute fibronectin to the desired concentration. Optimum conditions for attachment are dependent on cell type and application. The typical coating concentration is 1 – 5 ug/cm2.Fibronectin coating protocol, products, and FAQs.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique