A0487
Argatroban monohydrate
≥98% (HPLC)
Synonyme(s) :
(2R,4R)-1-[(2S)-5-[(Aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2-piperidinecarboxylic Acid, Argipidine, MQPA
About This Item
Produits recommandés
Niveau de qualité
Essai
≥98% (HPLC)
Forme
powder
Conditions de stockage
desiccated
Couleur
white to off-white
Solubilité
DMSO: ≥20 mg/mL
Auteur
Baxter
Température de stockage
2-8°C
Chaîne SMILES
[S](=O)(=O)(NC(CCCNC(=N)N)C(=O)N3[C@H](C[C@@H](CC3)C)C(=O)O)c1c2c(ccc1)CC(CN2)C.O
InChI
1S/C23H36N6O5S.H2O/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19;/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26);1H2/t14-,15?,17?,18-;/m1./s1
Clé InChI
AIEZTKLTLCMZIA-LINCPPCXSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The search for a functional thrombin receptor, using expression cloning methods, led to the discovery of a G protein-coupled receptor that mediates the actions of thrombin on platelets and endothelial cells.
Contenu apparenté
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique



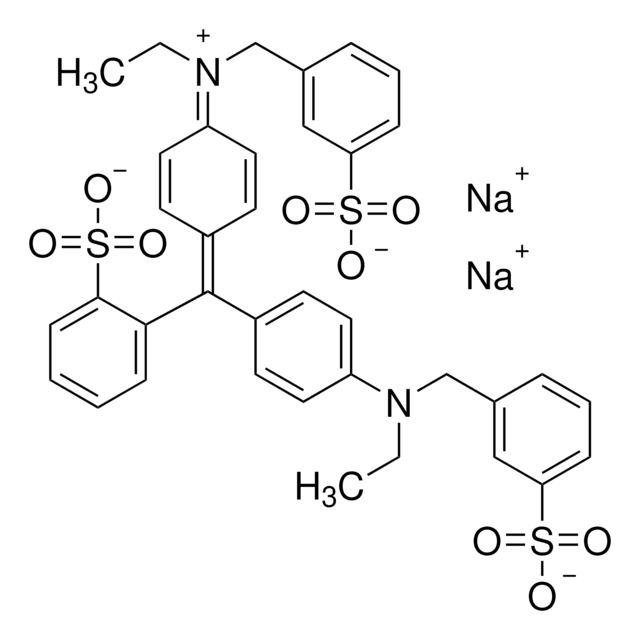

![PPACK, Dihydrochloride PPACK, Dihydrochloride, CAS 82188-90-7, is an extremely potent and selective irreversible inhibitor of thrombin (Kobs/[I] = 10⁷M⁻¹S⁻¹). Reacts with thrombin in a 1:1 stoichiometry.](/deepweb/assets/sigmaaldrich/product/images/403/286/e04cdb4e-07b0-4353-b8e5-13b915eae16c/640/e04cdb4e-07b0-4353-b8e5-13b915eae16c.jpg)