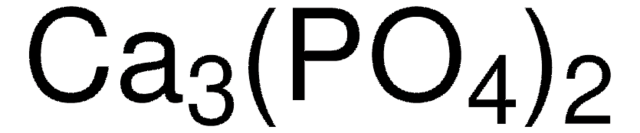49963
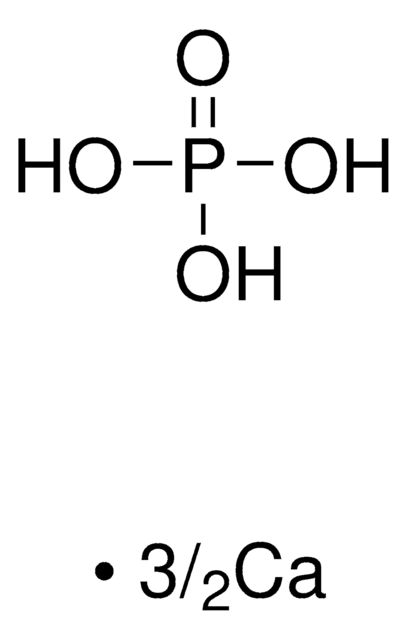
β-tri-Calcium phosphate
puriss. p.a., ≥98% β-phase basis (sintered Powder)
Synonyme(s) :
β-TCP, β-Tricalcium phosphate
About This Item
Produits recommandés
Qualité
puriss. p.a.
Niveau de qualité
Pureté
≥98% β-phase basis (sintered Powder)
Forme
powder
Impuretés
≤50 mg/kg total heavy metals as lead
≤500 mg/kg total sulfur as SO4 (ICP)
Traces d'anions
chloride (Cl-): ≤50 mg/kg
Traces de cations
As: ≤0.5 mg/kg
Ba: ≤20 mg/kg
Cd: ≤1 mg/kg
Co: ≤1 mg/kg
Cr: ≤10 mg/kg
Cu: ≤5 mg/kg
Hg: ≤0.5 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤20 mg/kg
InChI
1S/3Ca.2H3O4P/c;;;2*1-5(2,3)4/h;;;2*(H3,1,2,3,4)/q3*+2;;/p-6
Clé InChI
QORWJWZARLRLPR-UHFFFAOYSA-H
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Tungstosilicic Acid: A Promising Electrolyte for Redox Flow Battery.: This study explores the use of tungstosilicic acid (TSA) as an electrolyte in redox flow batteries (RFB). The research highlights TSA′s high energy density due to its multi-electron transfer capability, making it a promising candidate for enhancing the efficiency and power density of RFBs. This advancement is particularly relevant for large-scale energy storage systems integrating renewable energy technologies (Sharma et al., 2023).
- Heterogenization of a Tungstosilicic Acid Catalyst for Esterification of Bio-Oil Model Compound.: This paper explores the heterogenization of tungstosilicic acid by supporting it on silica-based materials for the esterification of bio-oil model compounds. The catalyst shows high activity and stability, indicating its potential for industrial applications in bio-oil upgrading (Prasertpong et al., 2022).
Remarque sur l'analyse
ß-TCP: >98% ; Hydroxylapatite: <1.0% ; a-TCP: <0%; TTCP: 0%
other Ca-P phases as Calcium pyrophosphate <1.0%
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique