C-045
Cannabidiol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonyme(s) :
CBD
About This Item
Produits recommandés
Qualité
certified reference material
Niveau de qualité
Forme
liquid
Caractéristiques
SNAP-N-SPIKE®, SNAP-N-SHOOT®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
Concentration
1.0 mg/mL in methanol
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
cannabis testing
cannabis testing
Format
single component solution
Température de stockage
−20°C
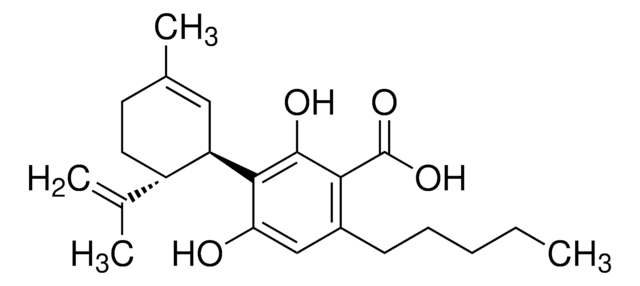
Chaîne SMILES
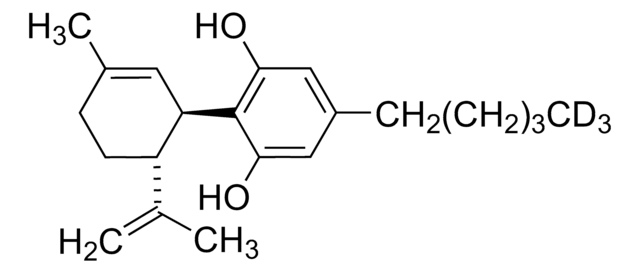
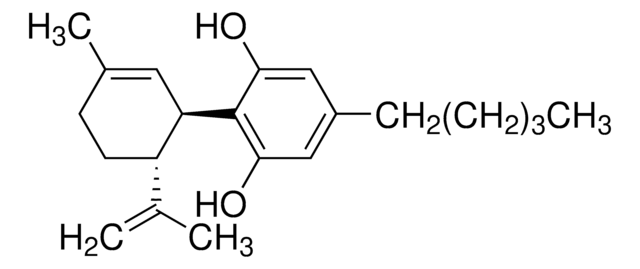
CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1
InChI
1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1
Clé InChI
QHMBSVQNZZTUGM-ZWKOTPCHSA-N
Informations sur le gène
human ... CNR1(1268)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Cannabidiol (CBD) is one of the important cannabinoids found in plants of the Cannabaceae family known for its non-psychoactive properties. It forms upon the decarboxylation of its precursor cannabidiolic acid in the presence of light or heat. It shows various therapeutic properties, such as antioxidative, anti-inflammatory, antimicrobial, neuroprotective, anxiolytic, and anticonvulsant.
Application
- Determination of four cannabinoids from an oil matrix using reversed-phase high-performance liquid chromatography (RP-HPLC) combined with a diode array detector (DAD)
- Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) based technique to measure cannabidiol, ∆9-tetrahydrocannabinol, and its metabolites in whole blood samples obtained from rats administered a high dose of cannabidiol
- Simultaneous determination of cannabidiol and tetrahydrocannabinol in different hemp oil-containing products using isocratic RP-HPLC coupled with UV detection
- Multi-analysis of hair samples for Δ9-tetrahydrocannabinol, cannabinol, and cannabidiol by liquid-liquid extraction (LLE) followed by gas chromatography-mass spectrometry based quantification
- Ultrasound-assisted extraction of cannabidiol from three different commercial topical products followed by GC-MS based determination
Caractéristiques et avantages
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes,Central nervous system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Listes réglementaires
Les listes réglementaires sont principalement fournies pour les produits chimiques. Seules des informations limitées peuvent être fournies ici pour les produits non chimiques. L'absence d'indication signifie qu'aucun des composants n'est répertorié. Il incombe à l'utilisateur de s'assurer de l'utilisation sûre et légale du produit.
EU REACH Annex XVII (Restriction List)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
High-performance liquid chromatography (HPLC) separation and quantitation of vitamin E acetate in commercially available vape products, in addition to simultaneous analysis of THC and CBD in the same run.
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabichromene solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
We offer a portfolio of cannabinoid CRMs such as THC and THCA, available as single component solutions or mixes, certified in accordance with ISO 17034 and ISO/IEC 17025 for accurate cannabinoid profiling and potency testing.
A comprehensive high performance liquid chromatography-diode array detection (HPLC-DAD) workflow for the analysis of 14 cannabinoids in hemp bud extracts within 10 minutes using robust Chromolith® monolithic silica HPLC columns with low column back pressure.
Protocoles
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabidiolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromene solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Cannabigerolic acid solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Δ9-Tetrahydrocannabinolic acid A (THCA-A)
A 100 mg sample of dried cannabis sativa leaves was extracted with 50 mL of ethanol:water (50:50, v/v) for 15 mins in an ultrasonic bath.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromenic Acid (CBCA) solution, 1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL
Global Trade Item Number
| Référence | GTIN |
|---|---|
| C-045-1ML | 4061833444559 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique