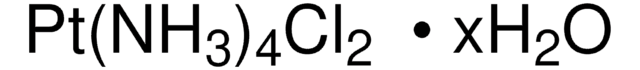520640
Platinum(IV) chloride
≥99.9% trace metals basis
Synonyme(s) :
Platinum tetrachloride
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥99.9% trace metals basis
Forme
powder
Composition
Pt, 55-58%
Pertinence de la réaction
reagent type: catalyst
core: platinum
Impuretés
≤1000.0 ppm Trace Metal Analysis
Pf
370 °C (dec.) (lit.)
Densité
4.303 g/mL at 25 °C (lit.)
Chaîne SMILES
Cl[Pt](Cl)(Cl)Cl
InChI
1S/4ClH.Pt/h4*1H;/q;;;;+4/p-4
Clé InChI
FBEIPJNQGITEBL-UHFFFAOYSA-J
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Platinumnanoparticles can be synthesized by reacting platinum (IV) chloride with Cyanobacteriumin an environmentally friendly method.
It can also be used as a dual catalyst forstereo- and regioselective glycosidations. Due to its ligand-bindingcapacity, platinum (IV) chloridehas an affinity for the glycosyl acceptor hydroxy group and forms the glycosidationproduct in β-configuration. It also enhances nucleophilicity differencesbetween hydroxy groups allowing regioselective glycosidation.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique










![[Pd(OAc)2]3 99.98% trace metals basis](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)