480894
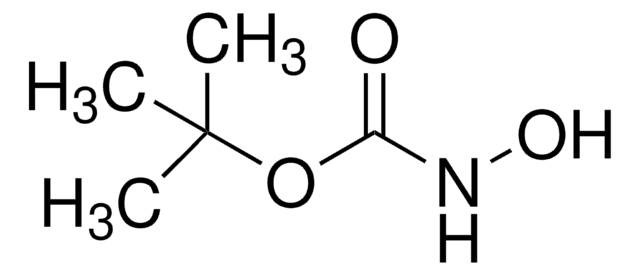
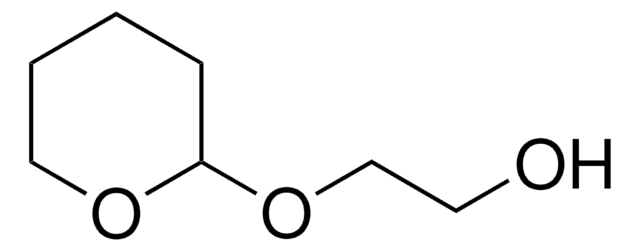
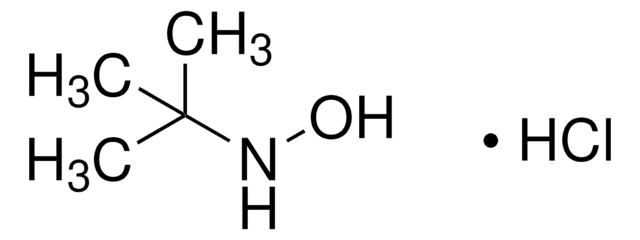
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine
96%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C5H11NO2
Numéro CAS:
Poids moléculaire :
117.15
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
96%
pb
81 °C/20 mmHg (lit.)
Pf
34-37 °C (lit.)
Groupe fonctionnel
ether
Chaîne SMILES
NOC1CCCCO1
InChI
1S/C5H11NO2/c6-8-5-3-1-2-4-7-5/h5H,1-4,6H2
Clé InChI
NLXXVSKHVGDQAT-UHFFFAOYSA-N
Catégories apparentées
Description générale
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine (OTX) is an O-substituted hydroxylamine. The coupling of OTX with alkaline gel electrophoresis has been reported to improve the process of detecting single strand breaks (SSBs) in DNA.
Application
O-(Tetrahydro-2H-pyran-2-yl)hydroxylamine may be used in the synthesis of 2-(5-bromothiophene-2-sulfonamido)-N-(tetrahydro-2H-pyran-2-yloxy)acetamides.
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
It may be used in the synthesis of the following potential histone deacetylase (HDAC) inhibitors:
- 2-[1-(naphthalene-2-sulfonyl)-heterocyclyl]-pyrimidine-5-carboxylic acid (tetrahydropyran-2-yloxy)-amides
- (E)-3-(2-benzyl-1-oxoisoindolin-6-yl)-N-(tetrahydro-2H-pyran-2-yloxy)acrylamide
- 3-(1-benzenesulfonyl-2,3-dihydro-1H-indol-5-yl)-N-hydroxy-acrylamide
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
179.6 °F - closed cup
Point d'éclair (°C)
82 °C - closed cup
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Chihiro Shinji et al.
Bioorganic & medicinal chemistry, 14(22), 7625-7651 (2006-08-01)
A series of hydroxamic acid derivatives bearing a cyclic amide/imide group as a linker and/or cap structure, prepared during our structural development studies based on thalidomide, showed class-selective potent histone deacetylase (HDAC)-inhibitory activity. Structure-activity relationship studies indicated that the steric
Patrick Angibaud et al.
European journal of medicinal chemistry, 40(6), 597-606 (2005-06-01)
A series of pyrimidyl-5-hydroxamic acids was prepared for evaluation as inhibitors of histone deacetylase (HDAC). Amino-2-pyrimidinyl can be used as a linker to provide HDAC inhibitors of good enzymatic potency.
Elisa Nuti et al.
European journal of medicinal chemistry, 46(7), 2617-2629 (2011-04-26)
Matrix metalloproteinases (MMPs) are important factors in gliomas since these enzymes facilitate invasion into the surrounding brain and participate in neovascularization. In particular, the gelatinases (MMP-2 and MMP-9), and more recently MMP-25, have been shown to be highly expressed in
Tetrahedron Letters, 45, 133-133 (2004)
Han-Li Huang et al.
PloS one, 7(8), e43645-e43645 (2012-08-29)
Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E028, a novel N-hydroxyacrylamide-derived HDAC inhibitor, inhibited human colorectal cancer HCT116 cell growth
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique