418218
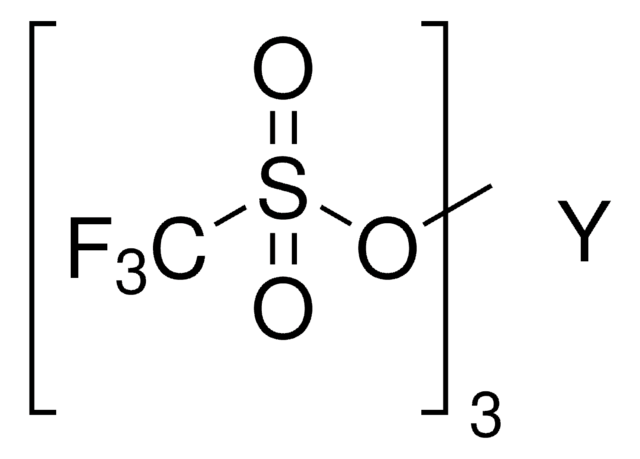
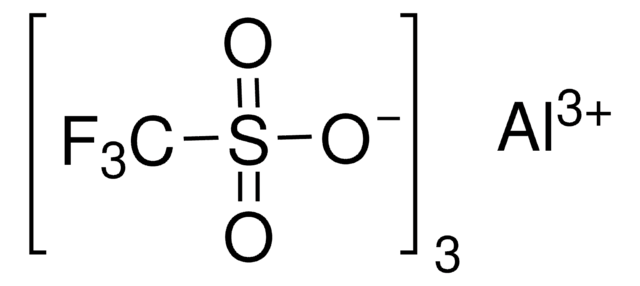
Scandium(III) triflate
99%
Synonyme(s) :
Sc(OTf)3, Scandium(III) trifluoromethanesulfonate, Trifluoromethanesulfonic acid scandium(III) salt
About This Item
Produits recommandés
Niveau de qualité
Pureté
99%
Forme
powder
Pertinence de la réaction
core: scandium
reagent type: catalyst
Chaîne SMILES
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
Clé InChI
HZXJVDYQRYYYOR-UHFFFAOYSA-K
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Hydrothiolation reaction of aromatic and aliphatic thiols.
- Selective two-electron reduction of O2 by ferrocene derivatives.
- Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
- Synthesis of β-cyanoketones.
- Combination with triethylsilane to reductively open functionalized pyranoside rings.
- The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique