155721
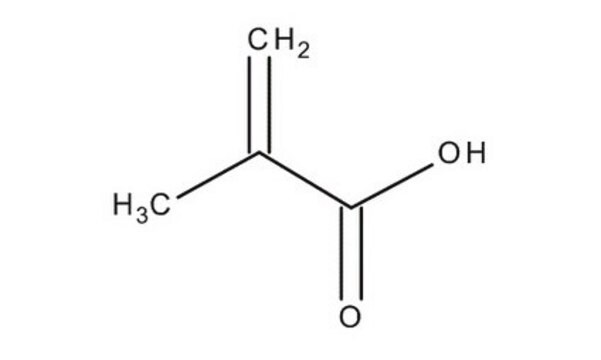
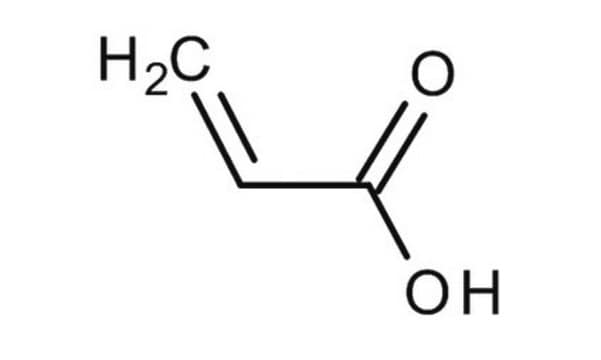
Methacrylic acid
contains 250 ppm MEHQ as inhibitor, 99%
Synonyme(s) :
2-Methacrylic acid, 2-Methylpropenoic acid
About This Item
Produits recommandés
Densité de vapeur
>3 (vs air)
Niveau de qualité
Pression de vapeur
1 mmHg ( 20 °C)
Pureté
99%
Forme
liquid
Température d'inflammation spontanée
752 °F
Contient
250 ppm MEHQ as inhibitor
Indice de réfraction
n20/D 1.431 (lit.)
pH
2.0-2.2 (20 °C, 100 g/L)
Point d'ébullition
163 °C (lit.)
Pf
12-16 °C (lit.)
Densité
1.015 g/mL at 25 °C (lit.)
Chaîne SMILES
C=C(C)C(O)=O
InChI
1S/C4H6O2/c1-3(2)4(5)6/h1H2,2H3,(H,5,6)
Clé InChI
CERQOIWHTDAKMF-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
152.6 °F - closed cup
Point d'éclair (°C)
67 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
(RAFT) Polymerization
Composites
Articles
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
By altering the physicochemical properties, smart or intelligent drug delivery systems can be designed to deliver therapeutic molecules on-demand. Learn more about the application of stimuli-responsive materials in drug delivery.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique