143685
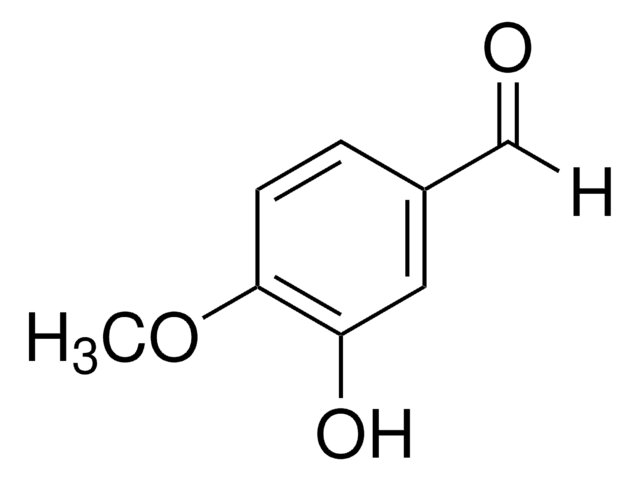
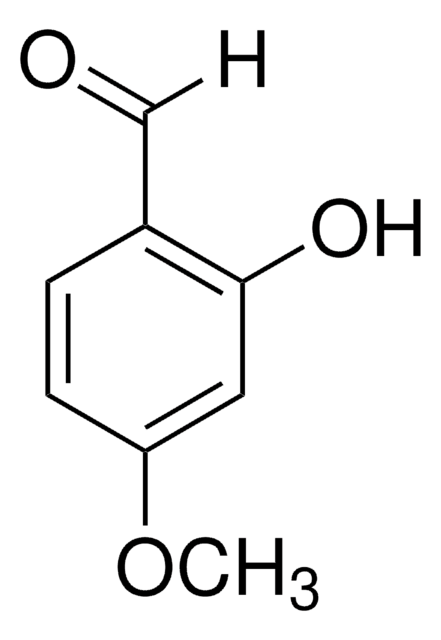
3-Hydroxy-4-methoxybenzaldehyde
99%
Synonyme(s) :
3-Hydroxyanisaldehyde, Isovanillin
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
HOC6H3(OCH3)CHO
Numéro CAS:
Poids moléculaire :
152.15
Numéro Beilstein :
1073021
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
99%
Point d'ébullition
179 °C/15 mmHg (lit.)
Pf
113-115 °C (lit.)
Groupe fonctionnel
aldehyde
Chaîne SMILES
[H]C(=O)c1ccc(OC)c(O)c1
InChI
1S/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
Clé InChI
JVTZFYYHCGSXJV-UHFFFAOYSA-N
Catégories apparentées
Description générale
3-Hydroxy-4-methoxybenzaldehyde on condensation with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide yields Schiff-bases. It undergoes condensation reaction with1-azabicyclo[2.2.2]octan-3-one to give (Z)-2-(3-hydroxy-4-methoxybenzylidene)-1-azabicyclo[2.2.2]octan-3-one.
Application
3-Hydroxy-4-methoxybenzaldehyde was used as starting reagent during the two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 and glycitein synthesis.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
>212.0 °F
Point d'éclair (°C)
> 100 °C
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Caroline Lang'at-Thoruwa et al.
Journal of natural products, 66(1), 149-151 (2003-01-25)
4-Methoxyresorcinol (3) was synthesized as the precursor for glycitein (6) synthesis by the oxidation of 3-hydroxy-4-methoxybenzaldehyde (1) to the aryl formate with H2O2 and a catalytic amount of SeO2. Glycitein (6) was synthesized by cyclization of 2,4,4'-trihydroxy-5-methoxydeoxybenzoin (5) with N,N-dimethylformamide
K Gaukroger et al.
The Journal of organic chemistry, 66(24), 8135-8138 (2001-11-28)
A high-yielding, two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 (1) has been devised. The method uses the Perkin condensation of 3,4,5-trimethoxyphenylacetic acid and 3-hydroxy-4-methoxybenzaldehyde followed by decarboxylation of the cinnamic acid intermediate using copper and quinoline. The iodine-catalyzed
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 11), o647-o649 (2003-11-08)
Crystals of the title compound, C(15)H(17)NO(3), were obtained from a condensation reaction of 3-hydroxy-4-methoxybenzaldehyde with 1-azabicyclo[2.2.2]octan-3-one and subsequent crystallization of the product from methanol. The title compound, containing a double bond that connects the azabicyclic ring system to the 3-hydroxy-4-methoxybenzylidene
Riyadh M Ahmed et al.
TheScientificWorldJournal, 2013, 754868-754868 (2013-09-13)
New monomeric cobalt and cadmium complexes with Schiff-bases, namely, N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]furan-2-carbohydrazide (L¹) and N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]thiophene-2-carbohydrazide (L²) are reported. Schiff-base ligands L¹ and L² were derived from condensation of 3-hydroxy-4-methoxybenzaldehyde (iso-vanillin) with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide, respectively. Complexes of the
Michael D Markey et al.
Organic letters, 9(17), 3255-3257 (2007-07-31)
The first total synthesis of santiagonamine (1) is achieved in 12 steps from isovanillin. A palladium-catalyzed Ullmann cross-coupling reaction and a photocyclization are the key steps in the synthesis.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique