D4287
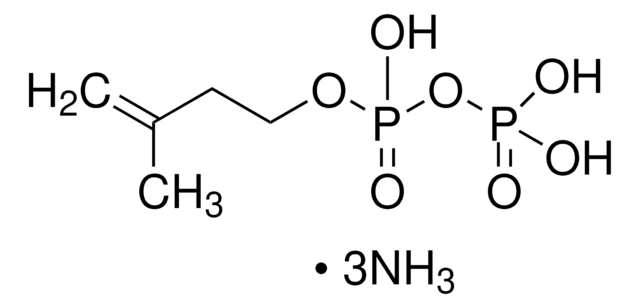
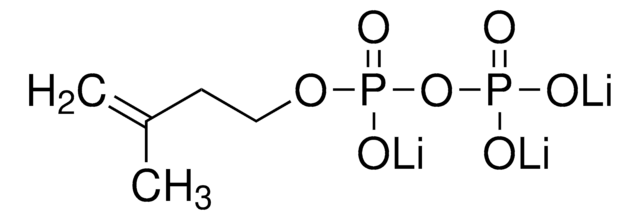
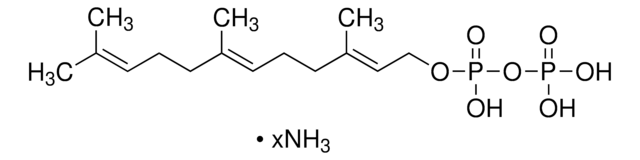
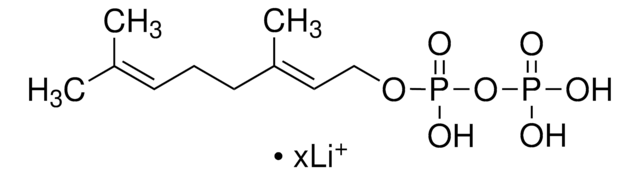
γ,γ-Dimethylallyl pyrophosphate triammonium salt
1 mg/mL in methanol (:aqueous 10 mM NH4OH (7:3)), ≥90% (TLC)
Synonym(s):
DMAPP
About This Item
Recommended Products
Quality Level
Assay
≥90% (TLC)
form
liquid
packaging
vial of 200 μg
concentration
1 mg/mL in methanol (:aqueous 10 mM NH4OH (7:3))
storage temp.
−20°C
SMILES string
N.N.N.C\C(C)=C\COP(O)(=O)OP(O)(O)=O
InChI
1S/C5H12O7P2.3H3N/c1-5(2)3-4-11-14(9,10)12-13(6,7)8;;;/h3H,4H2,1-2H3,(H,9,10)(H2,6,7,8);3*1H3
InChI key
VBUNGGIXIOHBHL-UHFFFAOYSA-N
Related Categories
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service