F6892
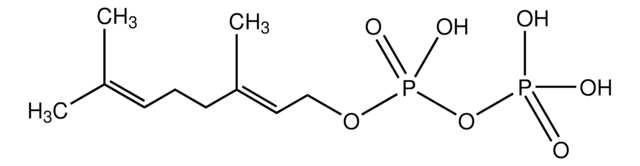
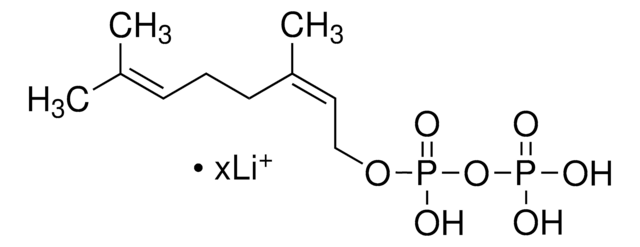
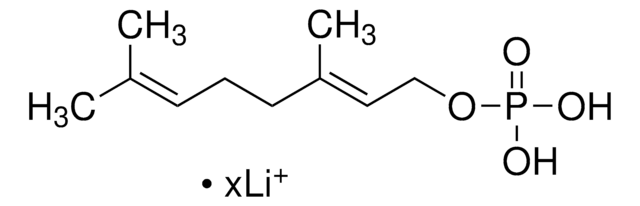
Farnesyl pyrophosphate ammonium salt
methanol:ammonia solution, ≥95% (TLC)
Synonym(s):
3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl pyrophosphate ammonium salt, FPP
About This Item
Recommended Products
Quality Level
Assay
≥95% (TLC)
form
methanol:ammonia solution
packaging
vial of 200 μg
storage temp.
−20°C
SMILES string
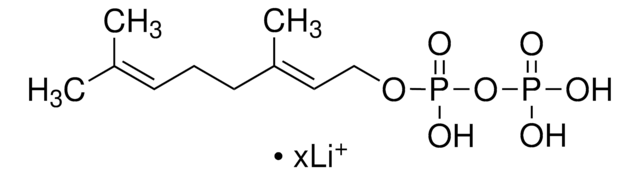
C\C(C)=C\CC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O
InChI
1S/C15H28O7P2/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-21-24(19,20)22-23(16,17)18/h7,9,11H,5-6,8,10,12H2,1-4H3,(H,19,20)(H2,16,17,18)/b14-9+,15-11+
InChI key
VWFJDQUYCIWHTN-YFVJMOTDSA-N
Gene Information
rat ... Fnta(25318)
General description
Application
- as a prenylation agonist in human osteogenic sarcoma cells in collagen-based cell invasion assays
- in the prenylation of the hepatocyte growth factor (HGF) in human umbilical vein endothelial cells (HUVECs)
- as a substrate in prenyltransferases assay in diatom Haslea ostrearia
Biochem/physiol Actions
Physical form
Actual concentration given on label
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
60.8 °F
Flash Point(C)
16 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service