110299
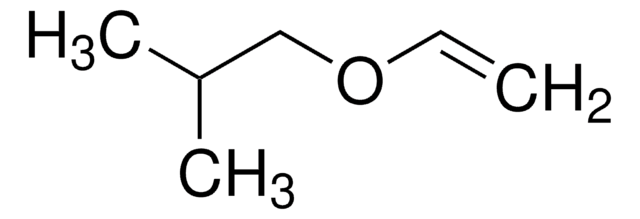
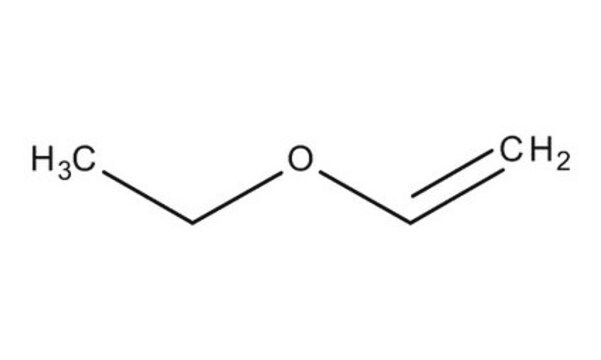
Butyl vinyl ether
contains 0.01% potassium hydroxide as stabilizer, 98%
Synonym(s):
(Butyloxy)ethylene, Vinyl butyl ether
About This Item
Recommended Products
vapor density
3.5 (vs air)
Quality Level
vapor pressure
42 mmHg ( 20 °C)
Assay
98%
autoignition temp.
437 °F
contains
0.01% potassium hydroxide as stabilizer
refractive index
n20/D 1.400 (lit.)
bp
94 °C (lit.)
mp
−92 °C (lit.)
density
0.774 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCOC=C
InChI
1S/C6H12O/c1-3-5-6-7-4-2/h4H,2-3,5-6H2,1H3
InChI key
UZKWTJUDCOPSNM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the synthesis of statistical copolymers through metallocene-mediated cationic polymerization. These statistical copolymers find applications in various fields which include adhesives, surface coatings, lubricants, greases, elastomers, melting compounds, fibers, and films.
- In the synthesis of poly(vinyl ether) plastics for specific applications in the optical industry. poly(vinyl ether) plastics can be utilized in the production of high-quality optical lenses for various applications, such as eyeglasses, camera lenses, and telescopes.
- In the synthesis of poly (butyl vinyl ether) adhesives for direct ink writing (DIW) 3D printing. The BVE-based adhesives exhibit viscoelastic properties, rheological properties, and printability which are advantageous for the additive build-up of extruded layers in DIW.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
10.4 °F - closed cup
Flash Point(C)
-12 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service