86857
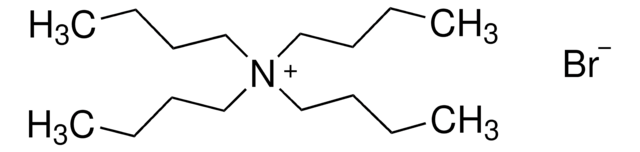
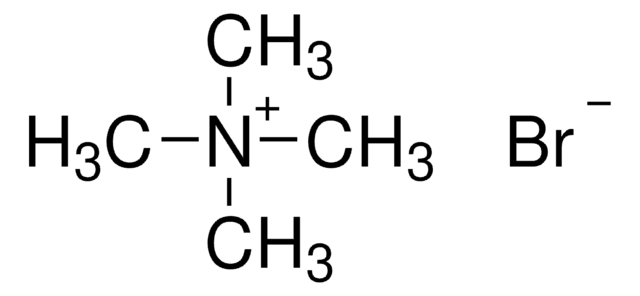
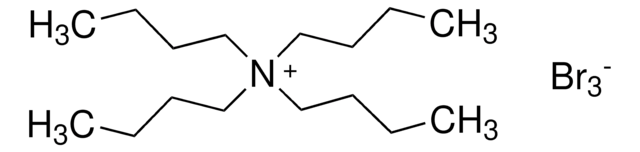
Tetrabutylammonium bromide
suitable for ion pair chromatography, LiChropur™, ≥99.0%
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
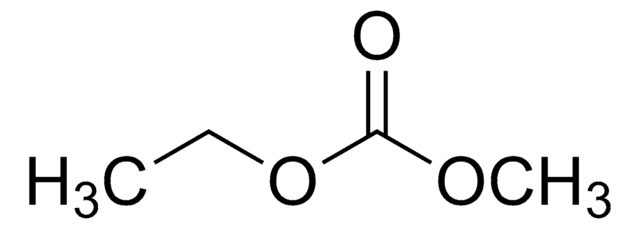
(CH3CH2CH2CH2)4N(Br)
CAS Number:
Molecular Weight:
322.37
Beilstein:
3570983
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NB.21
form:
crystals
Recommended Products
description
cationic
Quality Level
Assay
≥99.0% (AT)
≥99.0%
form
crystals
quality
LiChropur™
technique(s)
ion pair chromatography: suitable
mp
102-106 °C (lit.)
102-106 °C
λ
10 % in H2O
UV absorption
λ: 240 nm Amax: 0.04
λ: 250 nm Amax: 0.03
λ: 260 nm Amax: 0.02
λ: 500 nm Amax: 0.02
suitability
corresponds to standard for filter test
Looking for similar products? Visit Product Comparison Guide
General description
Tetrabutylammonium bromide, a quaternary ammonium compound widely used as a phase transfer catalyst. TBAB decreases the retention time and removes peak tailing by acting as an ion pair reagent during the chromatographic analysis of quaternary ammonium compounds. In the molten state, TBAB behaves like an ionic liquid, which is a promising green alternative to organic solvents in polymer synthesis.
Application
TBAB may have been used as ion-pairing reagent in phase-transfer catalytic extraction and derivatization procedure during micropollutant determination from water samples using GC technique.
Legal Information
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ion-pair single-drop microextraction versus phase-transfer catalytic extraction for the gas chromatographic determination of phenols as tosylated derivatives.
Fiamegos, Yiannis C., Ageliki-Panayota Kefala, and Constantine D. Stalikas.
Journal of Chromatography A, 1190.1, 44-51 (2008)
Novel biobased polyurethanes synthesized from nontoxic phenolic diol containing l-tyrosine moiety under green media.
Mallakpour S, et al.
Journal of Polymers and the Environment, 18.4, 685-695 (2010)
Direct polyamidation in molten tetrabutylammonium bromide: novel and efficient green media.
Mallakpour S and Yousefian H.
Polym. Bull., 60.2-3 , 191-198 (2008)
Effect of analogue ions in normal-phase ion-pair chromatography of quaternary ammonium compounds.
Bluhm LH and Li T.
Journal of Chromatographic Science, 37.8, 273-276 (1999)
Interfacial chemistry behavior of phase transfer catalysis for hydroxide ion initiated reactions.
Xia L, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 317.1, 747-750 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service