1724088
USP
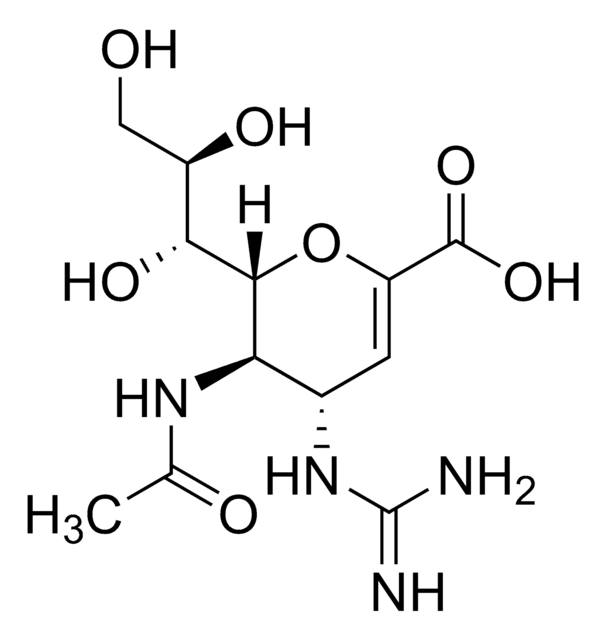
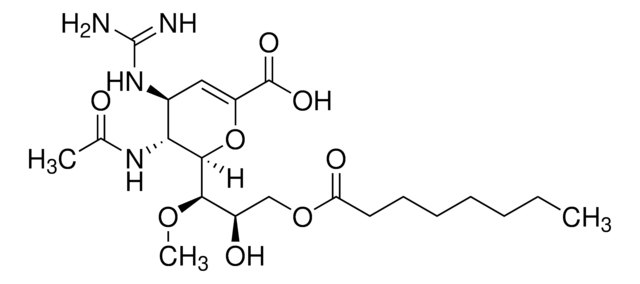
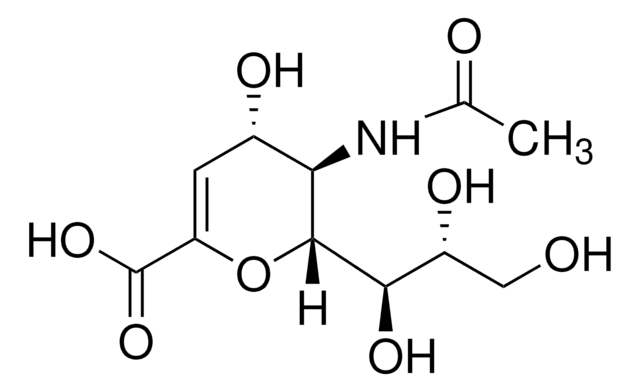
Zanamivir
United States Pharmacopeia (USP) Reference Standard
Sinónimos:
4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid, 4-Guanidino-Neu5Ac2en
About This Item
Productos recomendados
grado
pharmaceutical primary standard
familia API
zanamivir
fabricante / nombre comercial
USP
aplicaciones
pharmaceutical (small molecule)
formato
neat
temp. de almacenamiento
2-8°C
cadena SMILES
CC(=O)N[C@@H]1[C@@H](NC(N)=N)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O
InChI
1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1
Clave InChI
ARAIBEBZBOPLMB-UFGQHTETSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Nota de análisis
Otras notas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico