D9050
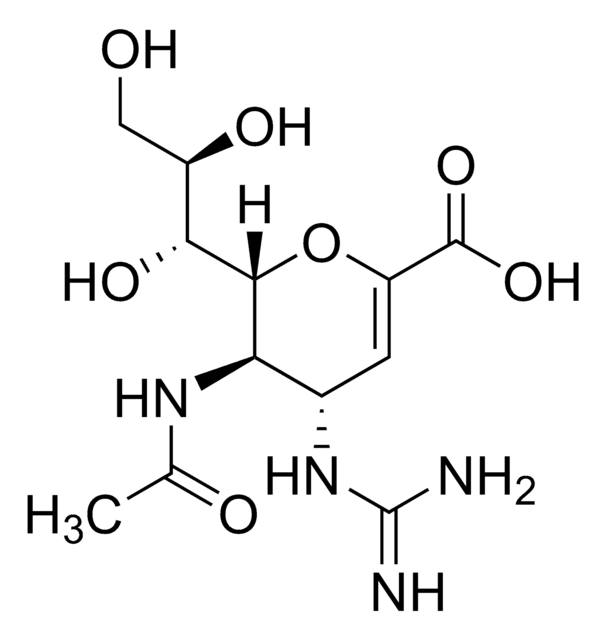
N-Acetyl-2,3-dehydro-2-deoxyneuraminic acid
≥93% (TLC)
Sinónimos:
2,3-Dehydro-2-deoxy-N-acetylneuraminic acid
About This Item
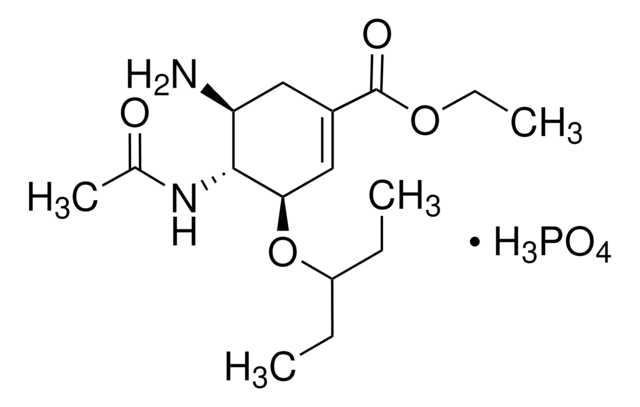
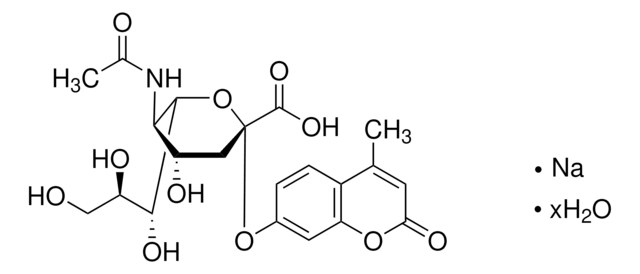
Productos recomendados
origen biológico
synthetic (organic)
Nivel de calidad
Ensayo
≥93% (TLC)
Formulario
powder
actividad óptica
[α]20/D 40.8 to 51.0 °, c = 0.66% (w/v) in water
color
white
solubilidad
H2O: soluble 50 mg/mL, clear, colorless
temp. de almacenamiento
−20°C
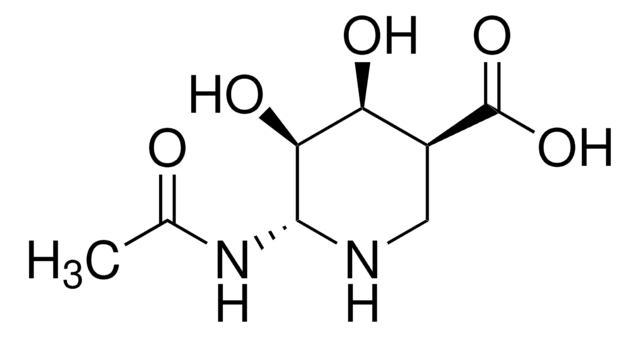
cadena SMILES
CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O
InChI
1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1
Clave InChI
JINJZWSZQKHCIP-UFGQHTETSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Acciones bioquímicas o fisiológicas
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico