U5625
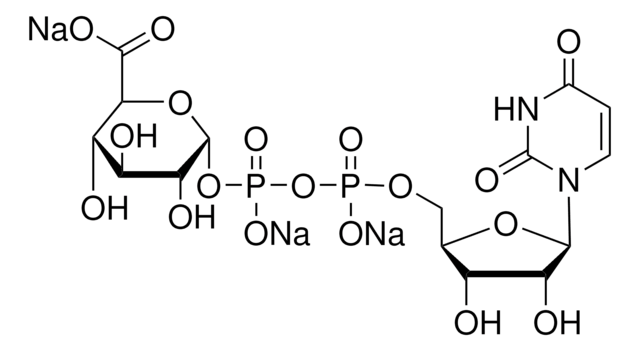
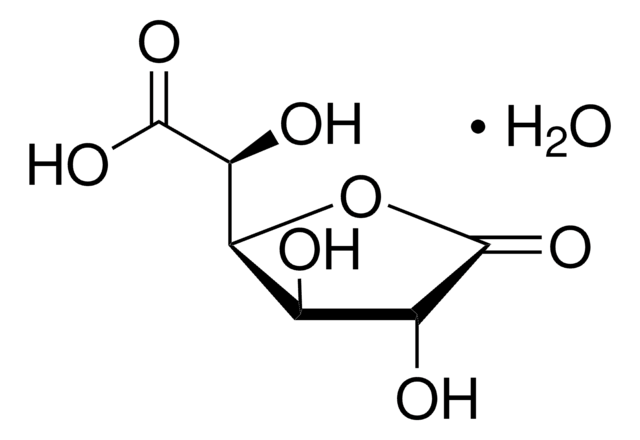
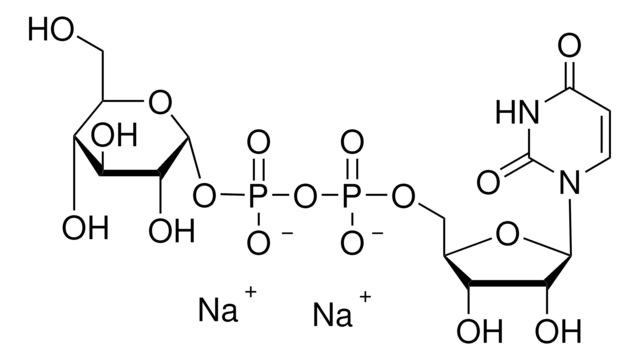
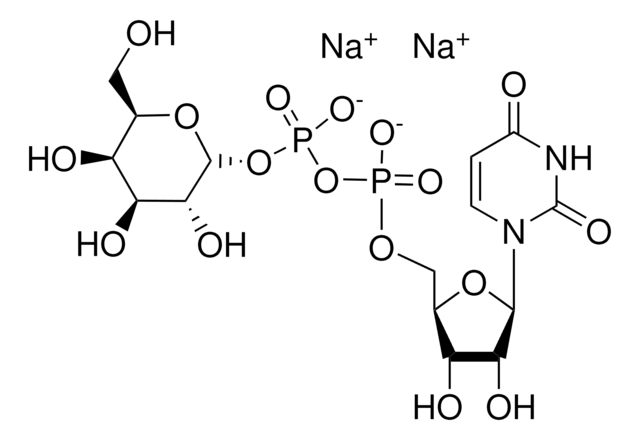
Uridine 5′-diphosphoglucuronic acid ammonium salt
98-100%
Sinónimos:
UDP-GlcA, UDPGA, Uridine-diphosphate-glucuronic acid ammonium salt, Uridine[5′]diphospho[1]-α-D-glucopyranosuronic acid ammonium salt
About This Item
Productos recomendados
origen biológico
Saccharomyces cerevisiae
enzyme from bovine liver (catalase)
enzyme from rabbit muscle (LDH)
Ensayo
98-100%
Formulario
powder
temp. de almacenamiento
−20°C
cadena SMILES
N.O[C@@H]1[C@@H](O)[C@H](O[C@@H]([C@H]1O)C(O)=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(=O)NC3=O
InChI
1S/C15H22N2O18P2.H3N/c18-5-1-2-17(15(26)16-5)12-9(22)6(19)4(32-12)3-31-36(27,28)35-37(29,30)34-14-10(23)7(20)8(21)11(33-14)13(24)25;/h1-2,4,6-12,14,19-23H,3H2,(H,24,25)(H,27,28)(H,29,30)(H,16,18,26);1H3/t4-,6-,7+,8+,9-,10-,11+,12-,14-;/m1./s1
Clave InChI
WMWKTCPGFOEPBD-YGIWDPDDSA-N
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The presence of multiple functional groups and stereocenters in complex carbohydrates makes them challenging targets for the organic chemist.
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico