U5127
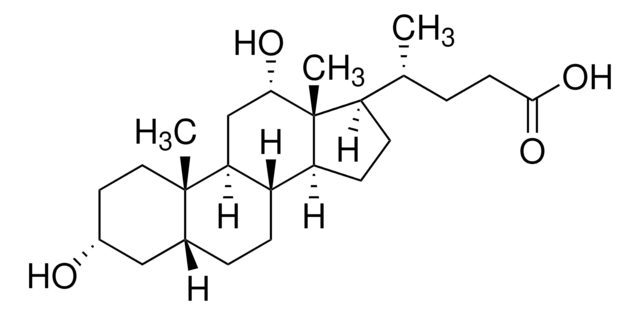
Ursodeoxycholic acid
≥99%
Sinónimos:
3α,7β-Dihydroxy-5β-cholan-24-oic acid, 5β-Cholan-24-oic acid-3α,7β-diol, 7β-Hydroxylithocholic acid, UDCS
About This Item
Productos recomendados
descripción
anionic detergent
Nivel de calidad
Análisis
≥99%
mol peso
392.57 g/mol
mp
203-204 °C (lit.)
cadena SMILES
C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C
InChI
1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1
Clave InChI
RUDATBOHQWOJDD-UZVSRGJWSA-N
Información sobre el gen
human ... NR1H4(9971)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Isobaric separation of bile acids and conjugates by LC-MS/MS on Ascentis® Express C18 column with excellent resolution and linearity.
Protocolos
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Contenido relacionado
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico