SRP5191
HSP90 α, His tagged human
recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
Sinónimos:
FLJ31884, HSP86, HSP90AA1, HSP90N, HSPC1, HSPCA, HSPCAL1, HSPCAL4, HSPN, Hsp89, Hsp90, LAP2
About This Item
Productos recomendados
origen biológico
human
recombinante
expressed in baculovirus infected Sf9 cells
Ensayo
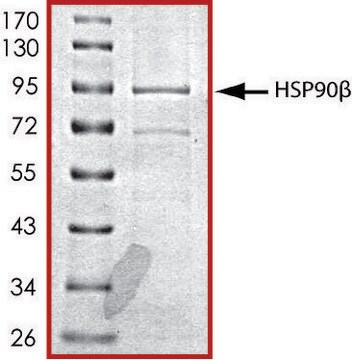
≥70% (SDS-PAGE)
Formulario
buffered aqueous glycerol solution
mol peso
~94 kDa
técnicas
cell culture | mammalian: suitable
solubilidad
water: soluble
idoneidad
suitable for molecular biology
Nº de acceso NCBI
Condiciones de envío
dry ice
temp. de almacenamiento
−70°C
Información sobre el gen
human ... HSP90AA1(3320)
Descripción general
Aplicación
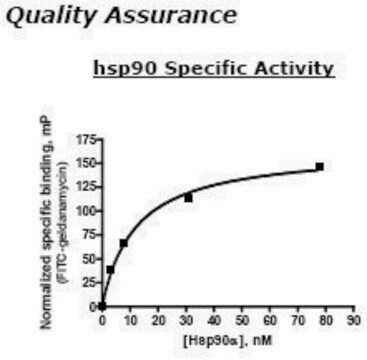
Acciones bioquímicas o fisiológicas
Forma física
Nota de preparación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Listados normativos
Los listados normativos se proporcionan para los productos químicos principalmente. Para los productos no químicos sólo se puede proporcionar información limitada. Si no hay ninguna entrada, significa que ninguno de los componentes está en la lista. Es obligación del usuario garantizar el uso seguro y legal del producto.
EU REACH Annex XVII (Restriction List)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico