SML0505
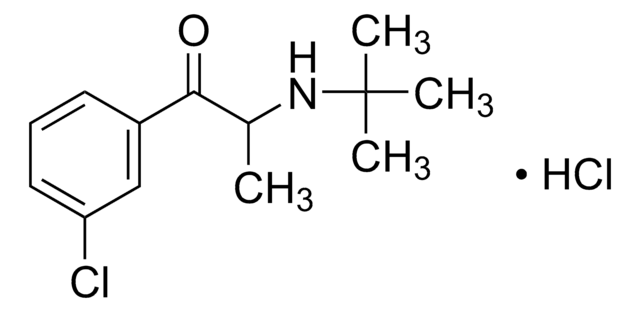
Tienilic Acid
≥98% (HPLC)
Sinónimos:
Ticrynafen, Tienylic acid, [2,3-Dichloro-4-(2-thienylcarbonyl)phenoxy]-acetic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C13H8Cl2O4S
Número de CAS:
Peso molecular:
331.17
Número CE:
Número MDL:
Código UNSPSC:
12161501
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
powder
color
white to beige
solubilidad
DMSO: 5 mg/mL (clear solution)
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
cadena SMILES
OC(=O)COc1ccc(c(Cl)c1Cl)C(=O)c2cccs2
InChI
1S/C13H8Cl2O4S/c14-11-7(13(18)9-2-1-5-20-9)3-4-8(12(11)15)19-6-10(16)17/h1-5H,6H2,(H,16,17)
Clave InChI
AGHANLSBXUWXTB-UHFFFAOYSA-N
Acciones bioquímicas o fisiológicas
Tienilic Acid (Ticrynafen) is a P450 inhibitor, a specific suicide substrate for CYP2C9 and CYP2C10. It was once used as a loop diuretic drug with uric acid-lowering (uricosuric) actvity, but was removed from market because of hepatotoxicity.
Tienilic Acid is a P450 inhibitor, a specific suicide substrate for CYP2C9 and CYP2C10.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Daisuke Satoh et al.
PloS one, 12(10), e0187072-e0187072 (2017-10-25)
The utility of HepG2 cells to assess drug metabolism and toxicity induced by chemical compounds is hampered by their low cytochrome P450 (CYP) activities. To overcome this limitation, we established HepG2 cell lines expressing major CYP enzymes involved in drug
Peter M Rademacher et al.
Chemical research in toxicology, 25(4), 895-903 (2012-02-15)
The uricosuric diuretic agent tienilic acid (TA) is a thiophene-containing compound that is metabolized by P450 2C9 to 5-OH-TA. A reactive metabolite of TA also forms a covalent adduct to P450 2C9 that inactivates the enzyme and initiates immune-mediated hepatic
Use of isotopes and LC-MS-ESI-TOF for mechanistic studies of tienilic acid metabolic activation.
M Belghazi et al.
Advances in experimental medicine and biology, 500, 139-144 (2002-01-05)
Hideo Takakusa et al.
Drug metabolism and disposition: the biological fate of chemicals, 36(5), 816-823 (2008-01-30)
The metabolic activation of a drug to an electrophilic reactive metabolite and its covalent binding to cellular macromolecules is considered to be involved in the occurrence of idiosyncratic drug toxicity (IDT). As a cellular defense system against oxidative and electrophilic
Takayoshi Nishiya et al.
Toxicology and applied pharmacology, 232(2), 280-291 (2008-08-19)
To investigate the hepatotoxic potential of tienilic acid in vivo, we administered a single oral dose of tienilic acid to Sprague-Dawley rats and performed general clinicopathological examinations and hepatic gene expression analysis using Affymetrix microarrays. No change in the serum
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico