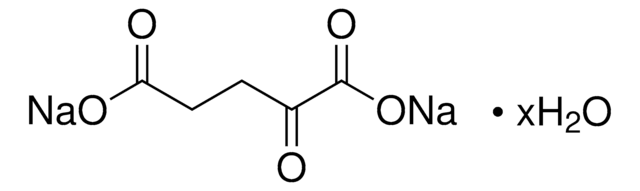
O4126
Oxaloacetic acid
≥97% (HPLC)
Sinónimos:
2-Oxosuccinic acid, Ketosuccinic acid, Oxalacetic acid, Oxobutanedioic acid
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥97% (HPLC)
Formulario
powder
solubilidad
H2O: 100 mg/mL, clear to slightly hazy, colorless to light yellow
temp. de almacenamiento
−20°C
cadena SMILES
OC(=O)CC(=O)C(O)=O
InChI
1S/C4H4O5/c5-2(4(8)9)1-3(6)7/h1H2,(H,6,7)(H,8,9)
Clave InChI
KHPXUQMNIQBQEV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Learn about monosaccharide biosynthesis and the metabolism of monosaccharides. A unit of a carbohydrate and the simplest form of a sugar, a monosaccharide cannot be hydrolyzed into a simpler compound.
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Protocolos
Enzymatic Assay of Malic Dehydrogenase (EC 1.1.1.37)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico