N8505
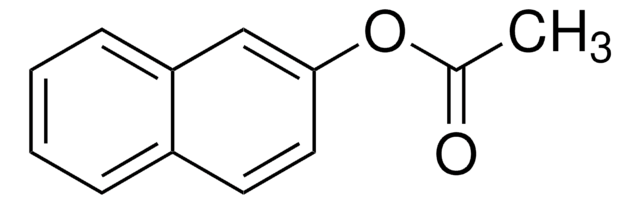
1-Naphthyl acetate
≥98% (C)
Sinónimos:
α-Naphthyl acetate
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥98% (C)
Formulario
crystals
mp
43-46 °C (lit.)
temp. de almacenamiento
−20°C
cadena SMILES
CC(=O)Oc1cccc2ccccc12
InChI
1S/C12H10O2/c1-9(13)14-12-8-4-6-10-5-2-3-7-11(10)12/h2-8H,1H3
Clave InChI
VGKONPUVOVVNSU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Dam. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F
Punto de inflamabilidad (°C)
113 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico