N1895
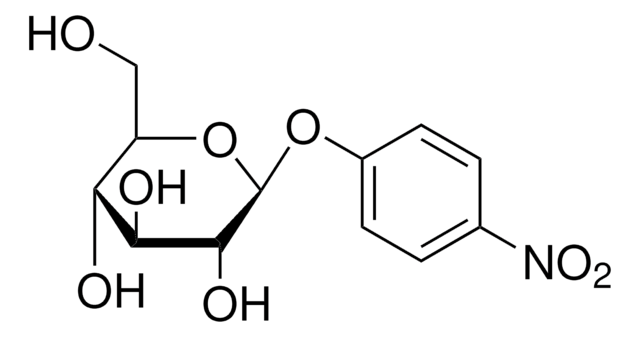
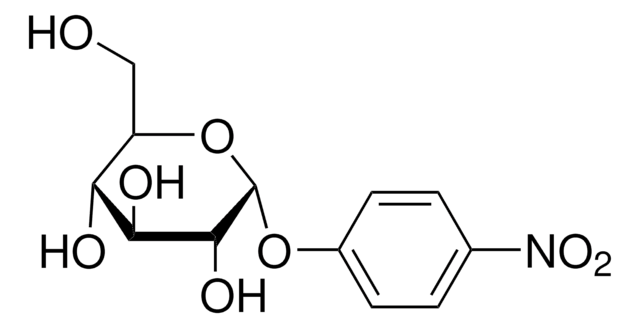
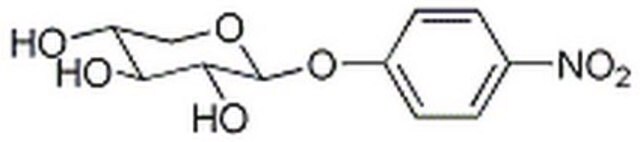
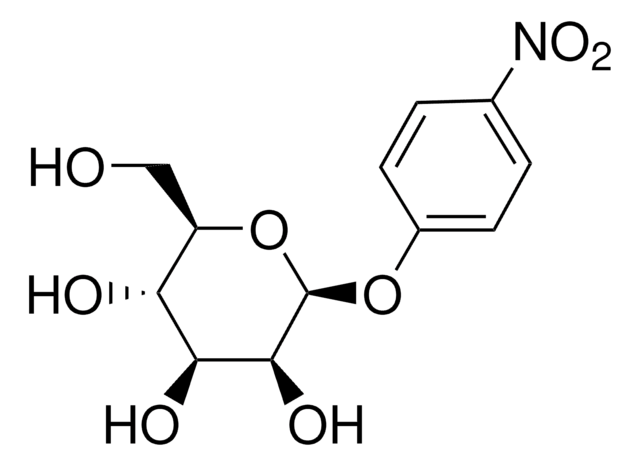
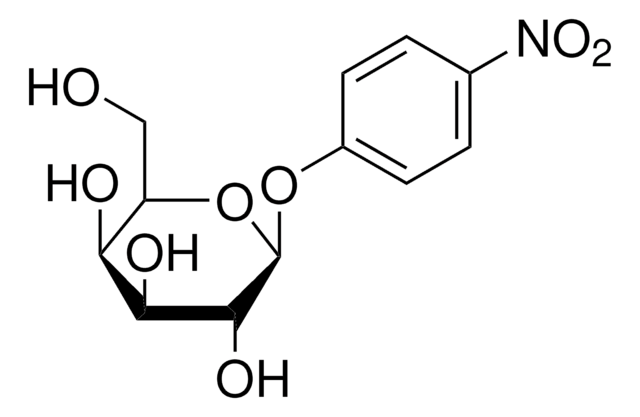
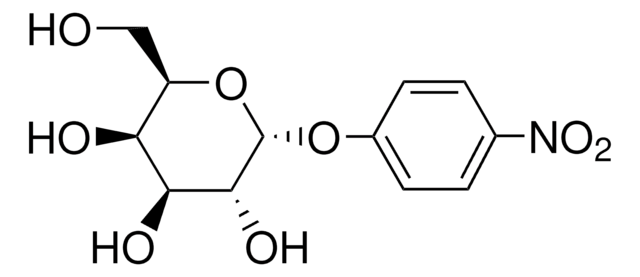
4-Nitrophenyl α-D-xylopyranoside
α-xylosidase substrate, ≥99% (HPLC), powder
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H13NO7
Número de CAS:
Peso molecular:
271.22
Número CE:
Número MDL:
Código UNSPSC:
12352204
ID de la sustancia en PubChem:
NACRES:
NA.32
Productos recomendados
Nombre del producto
4-Nitrophenyl α-D-xylopyranoside, α-xylosidase substrate
Nivel de calidad
Ensayo
≥99% (HPLC)
Formulario
powder
solubilidad
methanol: soluble 20 mg/mL, clear, colorless to faintly yellow
temp. de almacenamiento
−20°C
cadena SMILES
OC1COC(Oc2ccc(cc2)N(=O)=O)C(O)C1O
InChI
1S/C11H13NO7/c13-8-5-18-11(10(15)9(8)14)19-7-3-1-6(2-4-7)12(16)17/h1-4,8-11,13-15H,5H2
Clave InChI
MLJYKRYCCUGBBV-UHFFFAOYSA-N
Sustratos
Chromogenic substrate for α-xylosidase
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Masaki Yanagishita et al.
Kokubyo Gakkai zasshi. The Journal of the Stomatological Society, Japan, 73(1), 20-25 (2006-04-25)
Biosynthesis of proteoglycans and glycosaminoglycans in the presence of p-nitrophenyl-xyloside was studied using a primary rat ovarian granulosa cell culture system. Addition of p-nitrophenyl-xyloside into cell culture medium caused about a 700% increase of [³⁵S]sulfate incorporation (ED50 at 0.03 mM)
T Bravman et al.
FEBS letters, 495(1-2), 115-119 (2001-04-27)
A beta-xylosidase from Bacillus stearothermophilus T-6 was cloned, overexpressed in Escherichia coli and purified to homogeneity. Based on sequence alignment, the enzyme belongs to family 39 glycoside hydrolases, which itself forms part of the wider GH-A clan. The conserved Glu160
Mária Mastihubová et al.
Carbohydrate research, 339(7), 1353-1360 (2004-04-29)
Di-O-acetates and mono-O-acetates of 4-nitrophenyl beta-D-xylopyranoside were prepared by use of lipase PS-30. Polarity of organic solvents and reaction time affected the regioselectivity of the di-O-acetylation as well as the yields of monoacetates. The kinetics of acetyl groups migration in
Peter Biely et al.
Biochimica et biophysica acta, 1770(4), 565-570 (2007-01-31)
Positional specificity of NodB-like domain of a multidomain xylanase U from Clostridium thermocellum (CtAxe) was investigated. Of three monoacetates of 4-nitrophenyl beta-d-xylopyranoside the acetylxylan esterase domain showed a clear preference for the 2-acetate. Moreover, the enzyme was significantly activated by
Siyuan Li et al.
Histochemistry and cell biology, 139(1), 59-74 (2012-08-23)
Chondroitin/dermatan sulphate (CS/DS) sulphation motifs on cell and extracellular matrix proteoglycans (PGs) within stem/progenitor cell niches are involved in modulating cell phenotype during the development of many musculoskeletal connective tissues. Here, we investigate the importance of CS/DS chains and their
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico