N2132
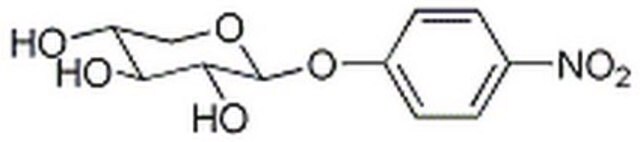
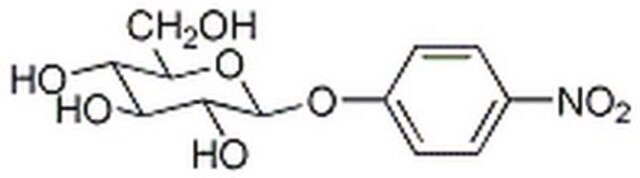
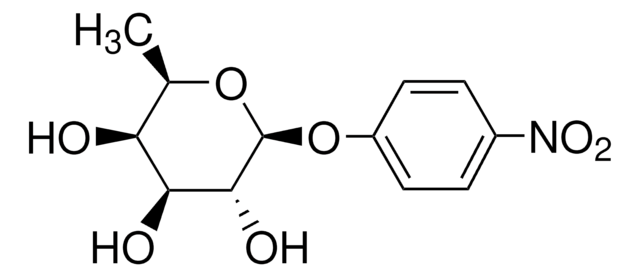
4-Nitrophenyl β-D-xylopyranoside
≥98%
Sinónimos:
PNPX
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥98%
Formulario
powder
puntuación de productos alternativos más sostenibles
old score: 100
new score: 51
Find out more about DOZN™ Scoring
características de los productos alternativos más sostenibles
Waste Prevention
Safer Solvents and Auxiliaries
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
solubilidad
methanol: 49.00-51.00 mg/mL
categoría alternativa más sostenible
temp. de almacenamiento
−20°C
cadena SMILES
O[C@@H]1CO[C@@H](Oc2ccc(cc2)[N+]([O-])=O)[C@H](O)[C@H]1O
InChI
1S/C11H13NO7/c13-8-5-18-11(10(15)9(8)14)19-7-3-1-6(2-4-7)12(16)17/h1-4,8-11,13-15H,5H2/t8-,9+,10-,11+/m1/s1
Clave InChI
MLJYKRYCCUGBBV-YTWAJWBKSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico