K3125
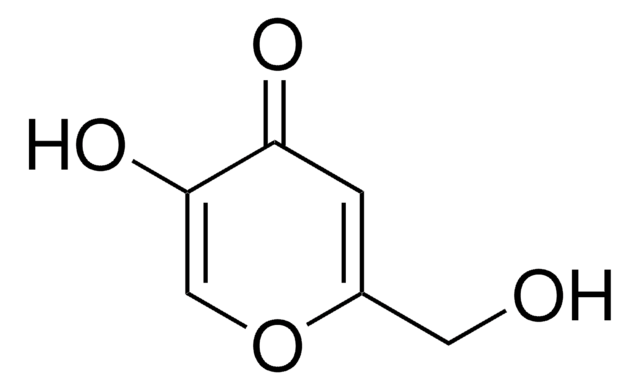
Kojic acid
≥98.5% (HPLC), powder, tyrosinase inhibitor
Sinónimos:
2-Hydroxymethyl-5-hydroxy-γ-pyrone, 5-Hydroxy-2-hydroxymethyl-4H-4-pyranone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H6O4
Número de CAS:
Peso molecular:
142.11
Beilstein:
120895
Número CE:
Número MDL:
Código UNSPSC:
12352106
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
Nombre del producto
Kojic acid,
Ensayo
≥98.5% (HPLC)
Nivel de calidad
Formulario
powder
mp
152-155 °C (lit.)
cadena SMILES
OCC1=CC(=O)C(O)=CO1
InChI
1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2
Clave InChI
BEJNERDRQOWKJM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Kojic acid has been used:
- as an inhibitor of tyrosinase in guinea pigs pigmented hyperopic (PH)
- as a reference inhibitor standard for screening tyrosinase inhibition
- as a positive control for inhibition of tyrosinase in B16F10 melanoma cells
Acciones bioquímicas o fisiológicas
Kojic acid is derived from some fungal species such as, Aspergillus, Acetobacter and Penicillium.. It halts melanin synthesis by inhibiting tyrosinase enzyme. It is used in the preparation of skin whitening cosmetics. However, kojic acid usage is minimal in cosmetics, as it induces skin irritation by its unstability and cytotoxic nature during long storage. It is an antioxidant and elicits radioprotective effects on chelating with manganese and zinc.
Tyrosinase inhibitor.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Effects of the Tyrosinase-Dependent Dopaminergic System on Refractive Error Development in Guinea Pigs
Jiang L, et al.
Investigative Ophthalmology & Visual Science, 59(11), 4631-4638 (2018)
Ka-Heng Lee et al.
European journal of medicinal chemistry, 44(8), 3195-3200 (2009-04-11)
A series of 46 curcumin related diarylpentanoid analogues were synthesized and evaluated for their anti-inflammatory, antioxidant and anti-tyrosinase activities. Among these compounds 2, 13 and 33 exhibited potent NO inhibitory effect on IFN-gamma/LPS-activated RAW 264.7 cells as compared to L-NAME
Kojic acid and its manganese and zinc complexes as potential radioprotective agents
Emami S, et al.
Bioorganic & Medicinal Chemistry Letters, 17(1), 45-48 (2007)
Xiao Hu et al.
Journal of natural products, 75(1), 82-87 (2011-12-15)
Two novel 2-arylbenzofuran dimers, morusyunnansins A and B (1 and 2), two new biflavonoids, morusyunnansins C and D (3 and 4), two new flavans, morusyunnansins E and F (5 and 6), and four known flavans (7-10) were isolated from the
Wei Yi et al.
European journal of medicinal chemistry, 46(9), 4330-4335 (2011-07-23)
Melanin play a major role in human skin protection and their biosynthesis is vital. Due to their color, they contribute to the skin pigmentation. Tyrosinase is a key enzyme involved in the first stage of melanin biosynthesis, it catalyzes the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico